Proteasomal chymotrypsin-like peptidase activity is required for essential functions of human monocyte-derived dendritic cells
- PMID: 17083604
- PMCID: PMC2265869
- DOI: 10.1111/j.1365-2567.2006.02487.x
Proteasomal chymotrypsin-like peptidase activity is required for essential functions of human monocyte-derived dendritic cells
Abstract
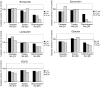
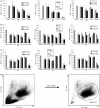
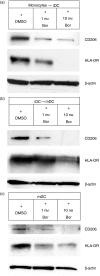
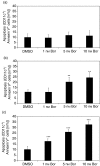
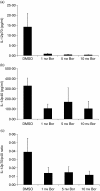
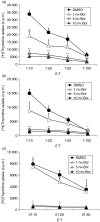
The ubiquitin-proteasome pathway is the principal system for extralysosomal protein degradation in eukaryotic cells, and is essential for the regulation and maintenance of basic cellular processes, including differentiation, proliferation, cell cycling, gene transcription and apoptosis. The 26S proteasome, a large multicatalytic protease complex, constitutes the system's proteolytic core machinery that exhibits different proteolytic activities residing in defined proteasomal subunits. We have identified proteasome inhibitors - bortezomib, epoxomicin and lactacystin - which selectively inhibit the proteasomal beta5 subunit-located chymotrypsin-like peptidase activity in human monocyte-derived dendritic cells (DCs). Inhibition of proteasomal chymotrypsin-like peptidase activity in immature and mature DCs impairs the cell-surface expression of CD40, CD86, CD80, human leucocyte antigen (HLA)-DR, CD206 and CD209, induces apoptosis, and impairs maturation of DCs, as demonstrated by decreased cell-surface expression of CD83 and lack of nuclear translocation of RelA and RelB. Inhibition of chymotrypsin-like peptidase activity abrogates macropinocytosis and receptor-mediated endocytosis of macromolecular antigens in immature DCs, and inhibits the synthesis of interleukin (IL)-12p70 and IL-12p40 in mature DCs. As a functional consequence, DCs fail to stimulate allogeneic CD4(+) and CD8(+) T cells and autologous CD4(+) T cells sufficiently in response to inhibition of chymotrypsin-like peptidase activity. Thus, proteasomal chymotrypsin-like peptidase activity is required for essential functions of human DCs, and inhibition of proteasomal chymotrypsin-like peptidase activity by selective inhibitors, or by targeting beta5 subunit expression, may provide a novel therapeutic strategy for suppression of deregulated and unwanted immune responses.
Figures

Similar articles
-
Proteasome inhibition suppresses essential immune functions of human CD4+ T cells.Immunology. 2008 Jun;124(2):234-46. doi: 10.1111/j.1365-2567.2007.02761.x. Epub 2008 Jan 23. Immunology. 2008. PMID: 18217957 Free PMC article.
-
Dendritic cell maturation stage determines susceptibility to the proteasome inhibitor bortezomib.Hum Immunol. 2007 Mar;68(3):147-55. doi: 10.1016/j.humimm.2006.12.005. Epub 2007 Jan 4. Hum Immunol. 2007. PMID: 17349869
-
Increased expression and altered subunit composition of proteasomes induced by continuous proteasome inhibition establish apoptosis resistance and hyperproliferation of Burkitt lymphoma cells.J Cell Biochem. 2008 Jan 1;103(1):270-83. doi: 10.1002/jcb.21405. J Cell Biochem. 2008. PMID: 17516511
-
Adaptive modification and flexibility of the proteasome system in response to proteasome inhibition.Biochim Biophys Acta. 2007 Sep;1773(9):1389-97. doi: 10.1016/j.bbamcr.2007.05.007. Epub 2007 May 25. Biochim Biophys Acta. 2007. PMID: 17582523 Review.
-
The proteasome and its inhibitors in immune regulation and immune disorders.Crit Rev Immunol. 2006;26(6):487-98. doi: 10.1615/critrevimmunol.v26.i6.20. Crit Rev Immunol. 2006. PMID: 17341190 Review.
Cited by
-
Immunomodulation in Administration of rAAV: Preclinical and Clinical Adjuvant Pharmacotherapies.Front Immunol. 2021 Apr 1;12:658038. doi: 10.3389/fimmu.2021.658038. eCollection 2021. Front Immunol. 2021. PMID: 33868303 Free PMC article. Review.
-
Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation.PLoS One. 2016 Jan 11;11(1):e0146553. doi: 10.1371/journal.pone.0146553. eCollection 2016. PLoS One. 2016. Retraction in: PLoS One. 2023 Mar 15;18(3):e0283354. doi: 10.1371/journal.pone.0283354. PMID: 26752181 Free PMC article. Retracted.
-
The ubiquitin-proteasome system and cardiovascular disease.Prog Mol Biol Transl Sci. 2012;109:295-346. doi: 10.1016/B978-0-12-397863-9.00009-2. Prog Mol Biol Transl Sci. 2012. PMID: 22727426 Free PMC article. Review.
-
The Deubiquitinase Inhibitor b-AP15 and Its Effect on Phenotype and Function of Monocyte-Derived Dendritic Cells.Neoplasia. 2019 Jul;21(7):653-664. doi: 10.1016/j.neo.2019.03.001. Epub 2019 May 25. Neoplasia. 2019. PMID: 31132676 Free PMC article.
-
Proteasome Levels and Activity in Pregnancies Complicated by Severe Preeclampsia and Hemolysis, Elevated Liver Enzymes, and Thrombocytopenia (HELLP) Syndrome.Hypertension. 2019 Jun;73(6):1308-1318. doi: 10.1161/HYPERTENSIONAHA.118.12437. Hypertension. 2019. PMID: 31067191 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Woltman AM, van Kooten C. Functional modulation of dendritic cells to suppress adaptive immune responses. J Leukoc Biol. 2003;73:428–41. - PubMed
-
- Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46. - PubMed
-
- Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nat Immunol. 2004;5:7–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

