Phosphorylation of HIV-1 Tat by CDK2 in HIV-1 transcription
- PMID: 17083724
- PMCID: PMC1636661
- DOI: 10.1186/1742-4690-3-78
Phosphorylation of HIV-1 Tat by CDK2 in HIV-1 transcription
Abstract
Background: Transcription of HIV-1 genes is activated by HIV-1 Tat protein, which induces phosphorylation of RNA polymerase II (RNAPII) C-terminal domain (CTD) by CDK9/cyclin T1. Earlier we showed that CDK2/cyclin E phosphorylates HIV-1 Tat in vitro. We also showed that CDK2 induces HIV-1 transcription in vitro and that inhibition of CDK2 expression by RNA interference inhibits HIV-1 transcription and viral replication in cultured cells. In the present study, we analyzed whether Tat is phosphorylated in cultured cells by CDK2 and whether Tat phosphorylation has a regulatory effect on HIV-1 transcription.
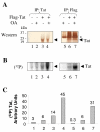
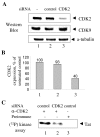
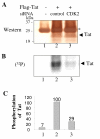
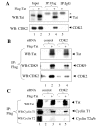
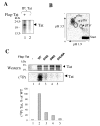
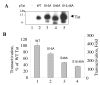
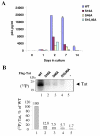
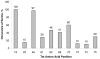
Results: We analyzed HIV-1 Tat phosphorylation by CDK2 in vitro and identified Ser16 and Ser46 residues of Tat as potential phosphorylation sites. Tat was phosphorylated in HeLa cells infected with Tat-expressing adenovirus and metabolically labeled with 32P. CDK2-specific siRNA reduced the amount and the activity of cellular CDK2 and significantly decreased phosphorylation of Tat. Tat co-migrated with CDK2 on glycerol gradient and co-immunoprecipitated with CDK2 from the cellular extracts. Tat was phosphorylated on serine residues in vivo, and mutations of Ser16 and Ser46 residues of Tat reduced Tat phosphorylation in vivo. Mutation of Ser16 and Ser46 residues of Tat reduced HIV-1 transcription in transiently transfected cells. The mutations of Tat also inhibited HIV-1 viral replication and Tat phosphorylation in the context of the integrated HIV-1 provirus. Analysis of physiological importance of the S16QP(K/R)19 and S46YGR49 sequences of Tat showed that Ser16 and Ser46 and R49 residues are highly conserved whereas mutation of the (K/R)19 residue correlated with non-progression of HIV-1 disease.
Conclusion: Our results indicate for the first time that Tat is phosphorylated in vivo; Tat phosphorylation is likely to be mediated by CDK2; and phosphorylation of Tat is important for HIV-1 transcription.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

