Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function
- PMID: 17083736
- PMCID: PMC1636062
- DOI: 10.1186/1471-2202-7-74
Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function
Abstract
Background: Neurodegeneration in Alzheimer's disease is associated with increased apoptosis and parallels increased levels of amyloid beta, which can induce neuronal apoptosis. Estrogen exposure prior to neurotoxic insult of hippocampal neurons promotes neuronal defence and survival against neurodegenerative insults including amyloid beta. Although all underlying molecular mechanisms of amyloid beta neurotoxicity remain undetermined, mitochondrial dysfunction, including altered calcium homeostasis and Bcl-2 expression, are involved in neurodegenerative vulnerability.
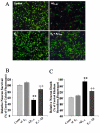
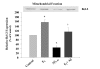
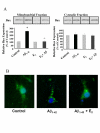
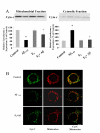
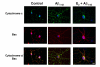
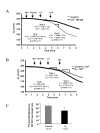
Results: In this study, we investigated the mechanism of 17beta-estradiol-induced prevention of amyloid beta-induced apoptosis of rat hippocampal neuronal cultures. Estradiol treatment prior to amyloid beta exposure significantly reduced the number of apoptotic neurons and the associated rise in resting intracellular calcium levels. Amyloid beta exposure provoked down regulation of a key antiapoptotic protein, Bcl-2, and resulted in mitochondrial translocation of Bax, a protein known to promote cell death, and subsequent release of cytochrome c. E2 pretreatment inhibited the amyloid beta-induced decrease in Bcl-2 expression, translocation of Bax to the mitochondria and subsequent release of cytochrome c. Further implicating the mitochondria as a target of estradiol action, in vivo estradiol treatment enhanced the respiratory function of whole brain mitochondria. In addition, estradiol pretreatment protected isolated mitochondria against calcium-induced loss of respiratory function.
Conclusion: Therefore, we propose that estradiol pretreatment protects against amyloid beta neurotoxicity by limiting mitochondrial dysfunction via activation of antiapoptotic mechanisms.
Figures


References
-
- Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. Journal of Neurochemistry. 1998;71:95–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

