Synthesis, antimalarial, antileishmanial, antimicrobial, cytotoxicity, and methemoglobin (MetHB) formation activities of new 8-quinolinamines
- PMID: 17084633
- PMCID: PMC4045844
- DOI: 10.1016/j.bmc.2006.10.036
Synthesis, antimalarial, antileishmanial, antimicrobial, cytotoxicity, and methemoglobin (MetHB) formation activities of new 8-quinolinamines
Abstract
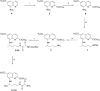
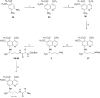
We report the synthesis, in vitro antiprotozoal (against Plasmodium and Leishmania), antimicrobial, cytotoxicity (Vero and MetHb-producing properties), and in vivo antimalarial activities of two series of 8-quinolinamines. N1-{4-[2-(tert-Butyl)-6-methoxy-8-quinolylamino]pentyl}-(2S/2R)-2-aminosubstitutedamides (21-33) and N1-[4-(4-ethyl-6-methoxy-5-pentyloxy-8-quinolylamino)pentyl]-(2S/2R)-2-aminosubstitutedamides (51-63) were synthesized in six steps from 6-methoxy-8-nitroquinoline and 4-methoxy-2-nitro-5-pentyloxyaniline, respectively. Several analogs displayed promising antimalarial activity in vitro against Plasmodium falciparum D6 (chloroquine-sensitive) and W2 (chloroquine-resistant) clones with high selectivity indices versus mammalian cells. The most promising analogs (21-24) also displayed potent antimalarial activity in vivo in a Plasmodium berghei-infected mouse model. Most interestingly, many analogs exhibited promising in vitro antileishmanial activity against Leishmania donovani promastigotes, and antimicrobial activities against a panel of pathogenic bacteria and fungi. Several analogs, notably 21-24, 26-32, and 60, showed less MetHb formation compared to primaquine indicating the potential of these compounds in 8-quinolinamine-based antimalarial drug development.
Figures
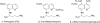
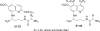
References
-
- World Health Organization Malaria Fact Sheet, No. 94, 2006. please see: http://www.who.int/mediacentre/factsheets/fs094/en/
-
- World Health Organization Leishmaniases Fact Sheet, No. 116, 2000. please see: http://www.who.int/mediacentre/factsheets/fs116/en/
-
- Overbye KM, Barrett JF. Drug Discov. Today. 2005;10:45. - PubMed
-
- Neu HC. Science. 1992;257:1064. - PubMed
-
- Vangapandu S, Jain M, Kaur K, Patil P, Patel SR, Jain R. Med. Res. Rev. 2006 DOI: 10.1002/med.20062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

