Regulation of the Drosophila distal antennal determinant spineless
- PMID: 17084833
- PMCID: PMC1876787
- DOI: 10.1016/j.ydbio.2006.09.044
Regulation of the Drosophila distal antennal determinant spineless
Abstract
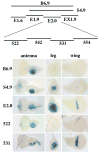
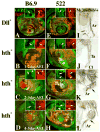
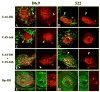
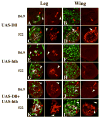
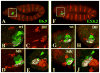
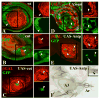
The transformation of antenna to leg is a classical model for understanding segmental fate decisions in Drosophila. The spineless (ss) gene encodes a bHLH-PAS transcription factor that plays a key role in specifying the identity of distal antennal segments. In this report, we identify the antennal disc enhancer of ss and then use enhancer-lacZ reporters to work out how ss antennal expression is regulated. The antennal determinants Distal-less (Dll) and homothorax (hth) are key activators of the antennal enhancer. Dll is required continuously and, when present at elevated levels, can activate the enhancer in regions devoid of hth expression. In contrast, homothorax (hth) is required only transiently both for activation of the enhancer and for specification of the aristal portion of the antenna. The antennal enhancer is repressed by cut, which determines its proximal limit of expression, and by ectopic Antennapedia (Antp). Repression by Antp is not mediated by hth, suggesting that ss may be a direct target of Antp. Finally, we show that ss+ is not a purely passive target of its regulators: ss+ partially represses hth in the third antennal segment and lies upstream of Dll in the development of the maxillary palp primordia.
Figures



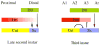
References
-
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. - PubMed
-
- Abzhanov A, Kaufman TC. Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol. 2000;227:673–689. - PubMed
-
- Angelini DR, Kaufman TC. Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects. Dev Biol. 2004;271:306–321. - PubMed
-
- Burgess EA, Duncan I. Direct control of antennal identity by the spineless-aristapedia gene of Drosophila. Mol Gen Genet. 1990;221:347–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

