DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint
- PMID: 17085480
- PMCID: PMC1635147
- DOI: 10.1101/gad.1482106
DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint
Abstract
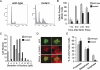
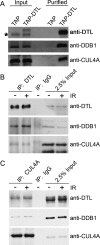
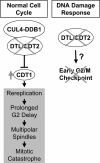
Checkpoint genes maintain genomic stability by arresting cells after DNA damage. Many of these genes also control cell cycle events in unperturbed cells. By conducting a screen for checkpoint genes in zebrafish, we found that dtl/cdt2 is an essential component of the early, radiation-induced G2/M checkpoint. We subsequently found that dtl/cdt2 is required for normal cell cycle control, primarily to prevent rereplication. Both the checkpoint and replication roles are conserved in human DTL. Our data indicate that the rereplication reflects a requirement for DTL in regulating CDT1, a protein required for prereplication complex formation. CDT1 is degraded in S phase to prevent rereplication, and following DNA damage to prevent origin firing. We show that DTL associates with the CUL4-DDB1 E3 ubiquitin ligase and is required for CDT1 down-regulation in unperturbed cells and following DNA damage. The cell cycle defects of Dtl-deficient zebrafish are suppressed by reducing Cdt1 levels. In contrast, the early G2/M checkpoint defect appears to be Cdt1-independent. Thus, DTL promotes genomic stability through two distinct mechanisms. First, it is an essential component of the CUL4-DDB1 complex that controls CDT1 levels, thereby preventing rereplication. Second, it is required for the early G2/M checkpoint.
Figures



References
-
- Angers, S., Li, T., Yi, X., MacCoss, M.J., Moon, R.T., Zheng, N. Molecular architecture and assembly of the DDB1–CUL4A ubiquitin ligase machinery. Nature. 2006 doi:10.1038/nature05175. - PubMed
-
- Arias, E., Walter, J. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat. Cell Biol. 2005;8:84–90. - PubMed
-
- Bell, S., Dutta, A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002;71:333–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
