A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis
- PMID: 17085506
- PMCID: PMC1761974
- DOI: 10.1104/pp.106.091322
A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis
Abstract
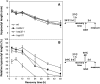
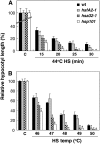
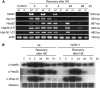
The expression of heat shock proteins (Hsps) induced by nonlethal heat treatment confers acquired thermotolerance (AT) to organisms against subsequent challenges of otherwise lethal temperature. After the stress signal is removed, AT gradually decays, with decreased Hsps during recovery. AT of sufficient duration is critical for sessile organisms such as plants to survive repeated heat stress in their environment, but little is known regarding its regulation. To identify potential regulatory components, we took a reverse genetics approach by screening for Arabidopsis (Arabidopsis thaliana) T-DNA insertion mutants that show decreased thermotolerance after a long recovery (2 d) under nonstress conditions following an acclimation heat treatment. Among the tested mutants corresponding to 48 heat-induced genes, only the heat shock transcription factor HsfA2 knockout mutant showed an obvious phenotype. Following pretreatment at 37 degrees C, the mutant line was more sensitive to severe heat stress than the wild type after long but not short recovery periods, and this could be complemented by the introduction of a wild-type copy of the HsfA2 gene. Quantitative hypocotyl elongation assay also revealed that AT decayed faster in the absence of HsfA2. Significant reduction in the transcript levels of several highly heat-inducible genes was observed in HsfA2 knockout plants after 4 h recovery or 2 h prolonged heat stress. Immunoblot analysis showed that Hsa32 and class I small Hsp were less abundant in the mutant than in the wild type after long recovery. Our results suggest that HsfA2 as a heat-inducible transactivator sustains the expression of Hsp genes and extends the duration of AT in Arabidopsis.
Figures




Similar articles
-
Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation.Plant Physiol. 2006 Apr;140(4):1297-305. doi: 10.1104/pp.105.074898. Epub 2006 Feb 24. Plant Physiol. 2006. PMID: 16500991 Free PMC article.
-
Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs.Plant J. 2009 Aug;59(3):387-99. doi: 10.1111/j.1365-313X.2009.03878.x. Epub 2009 Apr 2. Plant J. 2009. PMID: 19366428
-
Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress.Plant J. 2006 Nov;48(4):535-47. doi: 10.1111/j.1365-313X.2006.02889.x. Epub 2006 Oct 19. Plant J. 2006. PMID: 17059409
-
Complexity of the heat stress response in plants.Curr Opin Plant Biol. 2007 Jun;10(3):310-6. doi: 10.1016/j.pbi.2007.04.011. Epub 2007 May 4. Curr Opin Plant Biol. 2007. PMID: 17482504 Review.
-
HSFA2 orchestrates transcriptional dynamics after heat stress in Arabidopsis thaliana.Transcription. 2016 Aug 7;7(4):111-4. doi: 10.1080/21541264.2016.1187550. Epub 2016 Jul 6. Transcription. 2016. PMID: 27383578 Free PMC article. Review.
Cited by
-
Recent advances in plant thermomemory.Plant Cell Rep. 2021 Jan;40(1):19-27. doi: 10.1007/s00299-020-02604-1. Epub 2020 Sep 25. Plant Cell Rep. 2021. PMID: 32975635 Review.
-
Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome.BMC Plant Biol. 2016 Jul 20;16(1):164. doi: 10.1186/s12870-016-0850-0. BMC Plant Biol. 2016. PMID: 27439426 Free PMC article.
-
Use of heat stress responsive gene expression levels for early selection of heat tolerant cabbage (Brassica oleracea L.).Int J Mol Sci. 2013 Jun 4;14(6):11871-94. doi: 10.3390/ijms140611871. Int J Mol Sci. 2013. PMID: 23736694 Free PMC article.
-
Overexpression of Heat Shock Factor Gene HsfA3 Increases Galactinol Levels and Oxidative Stress Tolerance in Arabidopsis.Mol Cells. 2016 Jun 30;39(6):477-83. doi: 10.14348/molcells.2016.0027. Epub 2016 Apr 25. Mol Cells. 2016. PMID: 27109422 Free PMC article.
-
Proteome Analysis of the ROF-FKBP Mutants Reveals Functional Relations among Heat Stress Responses, Plant Development, and Protein Quality Control during Heat Acclimation in Arabidopsis thaliana.ACS Omega. 2023 Dec 26;9(2):2391-2408. doi: 10.1021/acsomega.3c06773. eCollection 2024 Jan 16. ACS Omega. 2023. PMID: 38250364 Free PMC article.
References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
-
- Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, Mishra SK, Nover L, Port M, Scharf KD, et al (2004) Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 29 471–487 - PubMed
-
- Bharti K, von Koskull-Doring P, Bharti S, Kumar P, Tintschl-Korbitzer A, Treuter E, Nover L (2004) Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell 16 1521–1535 - PMC - PubMed
-
- Boscheinen O, Lyck R, Queitsch C, Treuter E, Zimarino V, Scharf KD (1997) Heat stress transcription factors from tomato can functionally replace HSF1 in the yeast Saccharomyces cerevisiae. Mol Gen Genet 255 322–331 - PubMed
-
- Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, Andres L, Jiang H, Zheng G, Qian M, et al (2002) Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet 31 276–278 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

