Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency
- PMID: 17085511
- PMCID: PMC1761973
- DOI: 10.1104/pp.106.088609
Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency
Abstract
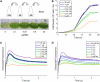
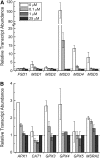
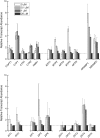
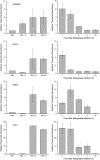
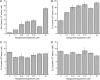
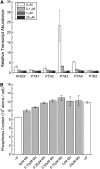
For photoheterotrophic growth, a Chlamydomonas reinhardtii cell requires at least 1.7 x 10(7) manganese ions in the medium. At lower manganese ion concentrations (typically <0.5 microm), cells divide more slowly, accumulate less chlorophyll, and the culture reaches stationary phase at lower cell density. Below 0.1 microm supplemental manganese ion in the medium, the cells are photosynthetically defective. This is accompanied by decreased abundance of D1, which binds the Mn(4)Ca cluster, and release of the OEE proteins from the membrane. Assay of Mn superoxide dismutase (MnSOD) indicates loss of activity of two isozymes in proportion to the Mn deficiency. The expression of MSD3 through MSD5, encoding various isoforms of the MnSODs, is up-regulated severalfold in Mn-deficient cells, but neither expression nor activity of the plastid Fe-containing superoxide dismutase is changed, which contrasts with the dramatically increased MSD3 expression and plastid MnSOD activity in Fe-deficient cells. Mn-deficient cells are selectively sensitive to peroxide but not methyl viologen or Rose Bengal, and GPXs, APX, and MSRA2 genes (encoding glutathione peroxidase, ascorbate peroxidase, and methionine sulfoxide reductase 2) are slightly up-regulated. Elemental analysis indicates that the Mn, Fe, and P contents of cells in the Mn-deficient cultures were reduced in proportion to the deficiency. A natural resistance-associated macrophage protein homolog and one of five metal tolerance proteins were induced in Mn-deficient cells but not in Fe-deficient cells, suggesting that the corresponding gene products may be components of a Mn(2+)-selective assimilation pathway.
Figures




References
-
- Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44 276–287 - PubMed
-
- Belouchi A, Kwan T, Gros P (1997) Cloning and characterization of the OsNramp family from Oryza sativa, a new family of membrane proteins possibly implicated in the transport of metal ions. Plant Mol Biol 33 1085–1092 - PubMed
-
- Bereczky Z, Wang HY, Schubert V, Ganal M, Bauer P (2003) Differential regulation of nramp and irt metal transporter genes in wild type and iron uptake mutants of tomato. J Biol Chem 278 24697–24704 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

