Glycosylation of Pseudomonas aeruginosa strain Pa5196 type IV pilins with mycobacterium-like alpha-1,5-linked d-Araf oligosaccharides
- PMID: 17085575
- PMCID: PMC1797228
- DOI: 10.1128/JB.01224-06
Glycosylation of Pseudomonas aeruginosa strain Pa5196 type IV pilins with mycobacterium-like alpha-1,5-linked d-Araf oligosaccharides
Abstract
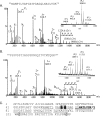
Pseudomonas aeruginosa is a gram-negative bacterium that uses polar type IV pili for adherence to various materials and for rapid colonization of surfaces via twitching motility. Within the P. aeruginosa species, five distinct alleles encoding variants of the structural subunit PilA varying in amino acid sequence, length, and presence of posttranslational modifications have been identified. In this work, a combination of mass spectrometry and nuclear magnetic resonance spectroscopy was used to identify a novel glycan modification on the pilins of the group IV strain Pa5196. Group IV pilins continued to be modified in a lipopolysaccharide (wbpM) mutant of Pa5196, showing that, unlike group I strains, the pilins of group IV are not modified with the O-antigen unit of the background strain. Instead, the pilin glycan was determined to be an unusual homo-oligomer of alpha-1,5-linked d-arabinofuranose (d-Araf). This sugar is uncommon in prokaryotes, occurring mainly in the cell wall arabinogalactan and lipoarabinomannan (LAM) polymers of mycobacteria, including Mycobacterium tuberculosis and Mycobacterium leprae. Antibodies raised against M. tuberculosis LAM specifically identified the glycosylated pilins from Pa5196, confirming that the glycan is antigenically, as well as chemically, identical to those of Mycobacterium. P. aeruginosa Pa5196, a rapidly growing strain of low virulence that expresses large amounts of glycosylated type IV pilins on its surface, represents a genetically tractable model system for elucidation of alternate pathways for biosynthesis of d-Araf and its polymerization into mycobacterium-like alpha-1,5-linked oligosaccharides.
Figures



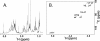

Similar articles
-
Pseudomonas aeruginosa D-arabinofuranose biosynthetic pathway and its role in type IV pilus assembly.J Biol Chem. 2011 Aug 12;286(32):28128-37. doi: 10.1074/jbc.M111.255794. Epub 2011 Jun 15. J Biol Chem. 2011. PMID: 21676874 Free PMC article.
-
Modification of Pseudomonas aeruginosa Pa5196 type IV Pilins at multiple sites with D-Araf by a novel GT-C family Arabinosyltransferase, TfpW.J Bacteriol. 2008 Nov;190(22):7464-78. doi: 10.1128/JB.01075-08. Epub 2008 Sep 19. J Bacteriol. 2008. PMID: 18805982 Free PMC article.
-
Evidence that biosynthesis of the second and third sugars of the archaellin Tetrasaccharide in the archaeon Methanococcus maripaludis occurs by the same pathway used by Pseudomonas aeruginosa to make a di-N-acetylated sugar.J Bacteriol. 2015 May;197(9):1668-80. doi: 10.1128/JB.00040-15. Epub 2015 Mar 2. J Bacteriol. 2015. PMID: 25733616 Free PMC article.
-
Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications.J Mol Microbiol Biotechnol. 2006;11(3-5):167-91. doi: 10.1159/000094053. J Mol Microbiol Biotechnol. 2006. PMID: 16983194 Review.
-
Pilins in gram-positive bacteria: A structural perspective.IUBMB Life. 2015 Jul;67(7):533-43. doi: 10.1002/iub.1400. Epub 2015 Jul 14. IUBMB Life. 2015. PMID: 26178080 Review.
Cited by
-
Type IV pilin proteins: versatile molecular modules.Microbiol Mol Biol Rev. 2012 Dec;76(4):740-72. doi: 10.1128/MMBR.00035-12. Microbiol Mol Biol Rev. 2012. PMID: 23204365 Free PMC article. Review.
-
Two isoforms of Geobacter sulfurreducens PilA have distinct roles in pilus biogenesis, cytochrome localization, extracellular electron transfer, and biofilm formation.J Bacteriol. 2012 May;194(10):2551-63. doi: 10.1128/JB.06366-11. Epub 2012 Mar 9. J Bacteriol. 2012. PMID: 22408162 Free PMC article.
-
Pseudomonas aeruginosa D-arabinofuranose biosynthetic pathway and its role in type IV pilus assembly.J Biol Chem. 2011 Aug 12;286(32):28128-37. doi: 10.1074/jbc.M111.255794. Epub 2011 Jun 15. J Biol Chem. 2011. PMID: 21676874 Free PMC article.
-
The structure of PilA from Acinetobacter baumannii AB5075 suggests a mechanism for functional specialization in Acinetobacter type IV pili.J Biol Chem. 2019 Jan 4;294(1):218-230. doi: 10.1074/jbc.RA118.005814. Epub 2018 Nov 9. J Biol Chem. 2019. PMID: 30413536 Free PMC article.
-
Genetic and mass spectrometry analyses of the unusual type IV-like pili of the archaeon Methanococcus maripaludis.J Bacteriol. 2011 Feb;193(4):804-14. doi: 10.1128/JB.00822-10. Epub 2010 Nov 12. J Bacteriol. 2011. PMID: 21075925 Free PMC article.
References
-
- Aas, F. E., W. Egge-Jacobsen, H. C. Winther-Larsen, C. Lovold, P. G. Hitchen, A. Dell, and M. Koomey. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to LPS phosphoethanolamine transferases. J. Biol. Chem. 281:27712-27723. - PubMed
-
- Belanger, A. E., G. S. Besra, M. E. Ford, K. Mikusova, J. T. Belisle, P. J. Brennan, and J. M. Inamine. 1996. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc. Natl. Acad. Sci. USA 93:11919-11924. - PMC - PubMed
-
- Benz, I., and M. A. Schmidt. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267-276. - PubMed
-
- Berg, S., J. Starbuck, J. B. Torrelles, V. D. Vissa, D. C. Crick, D. Chatterjee, and P. J. Brennan. 2005. Roles of conserved proline and glycosyltransferase motifs of EmbC in biosynthesis of lipoarabinomannan. J. Biol. Chem. 280:5651-5663. - PubMed
-
- Bock, K., J. Arnarp, and J. Lonngren. 1982. The preferred conformation of oligosaccharides derived from the complex-type carbohydrate portions of glycoproteins. Eur. J. Biochem. 129:171-178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

