Insights into transcription enhancer factor 1 (TEF-1) activity from the solution structure of the TEA domain
- PMID: 17085591
- PMCID: PMC1859914
- DOI: 10.1073/pnas.0607171103
Insights into transcription enhancer factor 1 (TEF-1) activity from the solution structure of the TEA domain
Abstract
Transcription enhancer factor 1 is essential for cardiac, skeletal, and smooth muscle development and uses its N-terminal TEA domain (TEAD) to bind M-CAT elements. Here, we present the first structure of TEAD and show that it is a three-helix bundle with a homeodomain fold. Structural data reveal how TEAD binds DNA. Using structure-function correlations, we find that the L1 loop is essential for cooperative loading of TEAD molecules on to tandemly duplicated M-CAT sites. Furthermore, using a microarray chip-based assay, we establish that known binding sites of the full-length protein are only a subset of DNA elements recognized by TEAD. Our results provide a model for understanding the regulation of genome-wide gene expression during development by TEA/ATTS family of transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
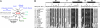
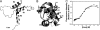




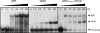
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

