Poly(3-hydroxybutyrate) granules at the early stages of formation are localized close to the cytoplasmic membrane in Caryophanon latum
- PMID: 17085698
- PMCID: PMC1796971
- DOI: 10.1128/AEM.01839-06
Poly(3-hydroxybutyrate) granules at the early stages of formation are localized close to the cytoplasmic membrane in Caryophanon latum
Abstract
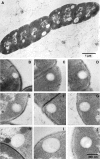
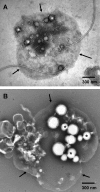
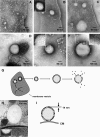
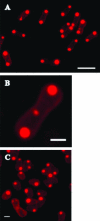
Localization of newly synthesized poly(3hydroxybutyrate) (PHB) granules was determined by confocal laser scanning fluorescence microscopy of Nile red-stained cells and by transmission electron microscopy (TEM). PHB granules of Nile red-stained living cells of Caryophanon latum at the early stages of PHB accumulation were frequently found at or close to the cytoplasmic membrane. TEM analysis of the same culture revealed electron-translucent globular structures resembling PHB granules that were nonrandomly distributed in the cell lumen but were frequently found at or close to the cytoplasmic membrane. Immunogold labeling using PHB-specific antiserum confirmed that the electron-translucent structures represented PHB granules. Electron microscopy examination of PHB granules after cell lysis revealed that PHB granules were often associated with membrane vesicles. Nonrandom localization of PHB granules was also found in Beijerinckia indica. Cells of this species harbored one PHB granule at each cell pole. Our results show that newly synthesized PHB granules often are close to or even in physical contact with the cytoplasmic membrane. Possible explanations for this unexpected finding and a hypothetical model of PHB granule formation in C. latum are discussed.
Figures


References
-
- Becking, J. 1992. The genus Beijerinckia, p. 2254-2267. In A. Balows, H. Trüper, M. Dworkin, W. Harder, and K. Schleifer (ed.), The prokaryotes, vol. III. Springer Verlag, New York, NY.
-
- Degelau, A., T. Scheper, J. Bailey, and C. Guske. 1995. Fluorometric measurements of poly-beta-hydroxybutyrate in Alcaligenes eutrophus by flow cytometry and spectrofluoromety. Appl. Microbiol. Biotechnol. 42:653-657.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

