Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs
- PMID: 17086186
- PMCID: PMC4698344
- DOI: 10.1038/ni1407
Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs
Abstract
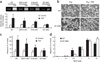
At sites of inflammation, ligation of leukocyte integrins is critical for the activation of cellular effector functions required for host defense. However, the signaling pathways linking integrin ligation to cellular responses are poorly understood. Here we show that integrin signaling in neutrophils and macrophages requires adaptors containing immunoreceptor tyrosine-based activation motifs (ITAMs). Neutrophils and macrophages lacking two ITAM-containing adaptor proteins, DAP12 and FcRgamma, were defective in integrin-mediated responses. Activation of the tyrosine kinase Syk by integrins required that DAP12 and FcRgamma were first phosphorylated by Src family kinases. Retroviral transduction of neutrophils and macrophages with wild-type and mutant Syk or DAP12 demonstrated that the Src homology 2 domains of Syk and the ITAM of DAP12 were required for integrin signaling. Our data show that integrin signaling for the activation of cellular responses in neutrophils and macrophages proceeds by an immunoreceptor-like mechanism.
Figures
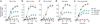



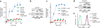


Comment in
-
Integrins and ITAMs: more than just good neighbors.Nat Immunol. 2006 Dec;7(12):1286-8. doi: 10.1038/ni1206-1286. Nat Immunol. 2006. PMID: 17110948 No abstract available.
References
-
- Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell. Signal. 1999;11:621–635. - PubMed
-
- Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving β2 integrins and selectin ligands. Curr. Opin. Hematol. 2002;9:30–35. - PubMed
-
- Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J. Allergy Clin. Immunol. 2004;113:620–626. - PubMed
-
- Thomas RM, et al. C-terminal SRC kinase controls acute inflammation and granulocyte adhesion. Immunity. 2004;20:181–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

