Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism
- PMID: 17087658
- PMCID: PMC1798442
- DOI: 10.1042/BJ20060665
Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism
Abstract
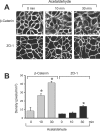
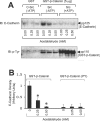
Interactions between E-cadherin, beta-catenin and PTP1B (protein tyrosine phosphatase 1B) are crucial for the organization of AJs (adherens junctions) and epithelial cell-cell adhesion. In the present study, the effect of acetaldehyde on the AJs and on the interactions between E-cadherin, beta-catenin and PTP1B was determined in Caco-2 cell monolayers. Treatment of cell monolayers with acetaldehyde induced redistribution of E-cadherin and beta-catenin from the intercellular junctions by a tyrosine phosphorylation-dependent mechanism. The PTPase activity associated with E-cadherin and beta-catenin was significantly reduced and the interaction of PTP1B with E-cadherin and beta-catenin was attenuated by acetaldehyde. Acetaldehyde treatment resulted in phosphorylation of beta-catenin on tyrosine residues, and abolished the interaction of beta-catenin with E-cadherin by a tyrosine kinase-dependent mechanism. Protein binding studies showed that the treatment of cells with acetaldehyde reduced the binding of beta-catenin to the C-terminal region of E-cadherin. Pairwise binding studies using purified proteins indicated that the direct interaction between E-cadherin and beta-catenin was reduced by tyrosine phosphorylation of beta-catenin, but was unaffected by tyrosine phosphorylation of E-cadherin-C. Treatment of cells with acetaldehyde also reduced the binding of E-cadherin to GST (glutathione S-transferase)-PTP1B. The pairwise binding study showed that GST-E-cadherin-C binds to recombinant PTP1B, but this binding was significantly reduced by tyrosine phosphorylation of E-cadherin. Acetaldehyde increased the phosphorylation of beta-catenin on Tyr-331, Tyr-333, Tyr-654 and Tyr-670. These results show that acetaldehyde induces disruption of interactions between E-cadherin, beta-catenin and PTP1B by a phosphorylation-dependent mechanism.
Figures








References
-
- Salaspuro M. P. Acetaldehyde, microbes, and cancer of the digestive tract. Crit. Rev. Clin. Lab. Sci. 2003;40:183–208. - PubMed
-
- Homann N., Tillonen J., Meurman J. H., Rintamaki H., Lindqvist C., Rautio M., Jousimies-Somer H., Salaspuro M. Increased salivary acetaldehyde levels in heavy drinkers and smokers: a microbiological approach to oral cavity cancer. Carcinogenesis. 2000;21:663–668. - PubMed
-
- Visapaa J. P., Tillonen J., Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 2002;37:322–326. - PubMed
-
- Poschl G., Seitz H. K. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. - PubMed
-
- Salaspuro M. Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann. Med. 1996;28:195–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

