ERAAP synergizes with MHC class I molecules to make the final cut in the antigenic peptide precursors in the endoplasmic reticulum
- PMID: 17088086
- PMCID: PMC2746443
- DOI: 10.1016/j.immuni.2006.09.012
ERAAP synergizes with MHC class I molecules to make the final cut in the antigenic peptide precursors in the endoplasmic reticulum
Abstract
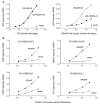
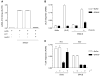
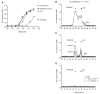
The major histocompatibility complex class I molecules display peptides (pMHC I) on the cell surface for immune surveillance by CD8(+) T cells. These peptides are generated by proteolysis of intracellular polypeptides by the proteasome in the cytoplasm and then in the endoplasmic reticulum (ER) by the ER aminopeptidase associated with antigen processing (ERAAP). To define the unknown mechanism of ERAAP function in vivo, we analyzed naturally processed peptides in cells with or without appropriate MHC I and ERAAP. In the absence of MHC I, ERAAP degraded the antigenic precursors in the ER. However, MHC I molecules could bind proteolytic intermediates and were essential for generation of the final peptide by ERAAP. Thus, ERAAP synergizes with MHC I to generate the final pMHC I repertoire.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



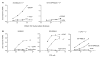
References
-
- Brouwenstijn N, Serwold T, Shastri N. MHC class I molecules can direct proteolytic cleavage of antigenic precursors in the endoplasmic reticulum. Immunity. 2001;15:95–104. - PubMed
-
- Elliott T, Townsend A, Cerundolo V. Antigen presentation: naturally processed peptides. Nature. 1990;348:195–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

