Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors
- PMID: 17088213
- PMCID: PMC1764219
- DOI: 10.1016/j.neuron.2006.08.034
Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors
Abstract
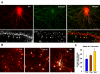
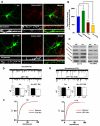
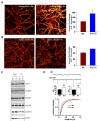
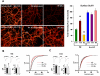
Homeostatic plasticity may compensate for Hebbian forms of synaptic plasticity, such as long-term potentiation (LTP) and depression (LTD), by scaling neuronal output without changing the relative strength of individual synapses. This delicate balance between neuronal output and distributed synaptic weight may be necessary for maintaining efficient encoding of information across neuronal networks. Here, we demonstrate that Arc/Arg3.1, an immediate-early gene (IEG) that is rapidly induced by neuronal activity associated with information encoding in the brain, mediates homeostatic synaptic scaling of AMPA type glutamate receptors (AMPARs) via its ability to activate a novel and selective AMPAR endocytic pathway. High levels of Arc/Arg3.1 block the homeostatic increases in AMPAR function induced by chronic neuronal inactivity. Conversely, loss of Arc/Arg3.1 results in increased AMPAR function and abolishes homeostatic scaling of AMPARs. These observations, together with evidence that Arc/Arg3.1 is required for memory consolidation, reveal the importance of Arc/Arg3.1's dynamic expression as it exerts continuous and precise control over synaptic strength and cellular excitability.
Figures

References
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. - PubMed
-
- Blackstone CD, Moss SJ, Martin LJ, Levey AI, Price DL, Huganir RL. Biochemical characterization and localization of a non-N-methyl-D-aspartate glutamate receptor in rat brain. J Neurochem. 1992;58:1118–1126. - PubMed
-
- Burke SN, Chawla MK, Penner MR, Crowell BE, Worley PF, Barnes CA, McNaughton BL. Differential encoding of behavior and spatial context in deep and superficial layers of the neocortex. Neuron. 2005;45:667–674. - PubMed
-
- Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol. 2001;63:847–869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

