Diversity of tRNA genes in eukaryotes
- PMID: 17088292
- PMCID: PMC1693877
- DOI: 10.1093/nar/gkl725
Diversity of tRNA genes in eukaryotes
Abstract
We compare the diversity of chromosomal-encoded transfer RNA (tRNA) genes from 11 eukaryotes as identified by tRNAScan-SE of their respective genomes. They include the budding and fission yeast, worm, fruit fly, fugu, chicken, dog, rat, mouse, chimp and human. The number of tRNA genes are between 170 and 570 and the number of tRNA isoacceptors range from 41 to 55. Unexpectedly, the number of tRNA genes having the same anticodon but different sequences elsewhere in the tRNA body (defined here as tRNA isodecoder genes) varies significantly (10-246). tRNA isodecoder genes allow up to 274 different tRNA species to be produced from 446 genes in humans, but only up to 51 from 275 genes in the budding yeast. The fraction of tRNA isodecoder genes among all tRNA genes increases across the phylogenetic spectrum. A large number of sequence differences in human tRNA isodecoder genes occurs in the internal promoter regions for RNA polymerase III. We also describe a systematic, ligation-based method to detect and quantify tRNA isodecoder molecules in human samples, and show differential expression of three tRNA isodecoders in six human tissues. The large number of tRNA isodecoder genes in eukaryotes suggests that tRNA function may be more diverse than previously appreciated.
Figures

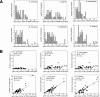


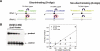
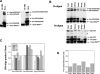
References
-
- Ikemura T., Ozeki H. Codon usage and transfer RNA contents: organism-specific codon-choice patterns in reference to the isoacceptor contents. Cold Spring Harb. Symp. Quant. Biol. 1983;47:1087–1097. - PubMed
-
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol. Biol. Evol. 1985;2:13–34. - PubMed
-
- Dong H., Nilsson L., Kurland C.G. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Mol. Biol. 1996;260:649–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

