Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation
- PMID: 17088319
- PMCID: PMC2242444
- DOI: 10.1110/ps.062465306
Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation
Abstract
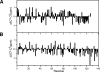
The synucleins are a family of intrinsically disordered proteins involved in various human diseases. alpha-Synuclein has been extensively characterized due to its role in Parkinson's disease where it forms intracellular aggregates, while gamma-synuclein is overexpressed in a majority of late-stage breast cancers. Despite fairly strong sequence similarity between the amyloid-forming regions of alpha- and gamma-synuclein, gamma-synuclein has only a weak propensity to form amyloid fibrils. We hypothesize that the different fibrillation tendencies of alpha- and gamma-synuclein may be related to differences in structural propensities. Here we have measured chemical shifts for gamma-synuclein and compared them to previously published shifts for alpha-synuclein. In order to facilitate direct comparison, we have implemented a simple new technique for re-referencing chemical shifts that we have found to be highly effective for both disordered and folded proteins. In addition, we have developed a new method that combines different chemical shifts into a single residue-specific secondary structure propensity (SSP) score. We observe significant differences between alpha- and gamma-synuclein secondary structure propensities. Most interestingly, gamma-synuclein has an increased alpha-helical propensity in the amyloid-forming region that is critical for alpha-synuclein fibrillation, suggesting that increased structural stability in this region may protect against gamma-synuclein aggregation. This comparison of residue-specific secondary structure propensities between intrinsically disordered homologs highlights the sensitivity of transient structure to sequence changes, which we suggest may have been exploited as an evolutionary mechanism for fast modulation of protein structure and, hence, function.
Figures


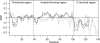
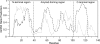

Similar articles
-
Residual structure, backbone dynamics, and interactions within the synuclein family.J Mol Biol. 2007 Sep 21;372(3):689-707. doi: 10.1016/j.jmb.2007.07.008. Epub 2007 Jul 17. J Mol Biol. 2007. PMID: 17681534 Free PMC article.
-
Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins.J Biol Chem. 2002 Apr 5;277(14):11970-8. doi: 10.1074/jbc.M109541200. Epub 2002 Jan 25. J Biol Chem. 2002. PMID: 11812782
-
A relationship between the transient structure in the monomeric state and the aggregation propensities of α-synuclein and β-synuclein.Biochemistry. 2014 Nov 25;53(46):7170-83. doi: 10.1021/bi5009326. Epub 2014 Nov 12. Biochemistry. 2014. PMID: 25389903 Free PMC article.
-
The synucleins.Genome Biol. 2002;3(1):REVIEWS3002. doi: 10.1186/gb-2001-3-1-reviews3002. Epub 2001 Dec 20. Genome Biol. 2002. PMID: 11806835 Free PMC article. Review.
-
Α-synuclein misfolding and Parkinson's disease.Biochim Biophys Acta. 2012 Feb;1822(2):261-85. doi: 10.1016/j.bbadis.2011.10.002. Epub 2011 Oct 12. Biochim Biophys Acta. 2012. PMID: 22024360 Review.
Cited by
-
Chemical shift prediction for denatured proteins.J Biomol NMR. 2013 Feb;55(2):201-9. doi: 10.1007/s10858-012-9702-x. Epub 2013 Jan 8. J Biomol NMR. 2013. PMID: 23297019 Free PMC article.
-
Structural characterization of KKT4, an unconventional microtubule-binding kinetochore protein.Structure. 2021 Sep 2;29(9):1014-1028.e8. doi: 10.1016/j.str.2021.04.004. Epub 2021 Apr 28. Structure. 2021. PMID: 33915106 Free PMC article.
-
Conformational changes relevant to channel activity and folding within the first nucleotide binding domain of the cystic fibrosis transmembrane conductance regulator.J Biol Chem. 2012 Aug 17;287(34):28480-94. doi: 10.1074/jbc.M112.371138. Epub 2012 Jun 21. J Biol Chem. 2012. PMID: 22722932 Free PMC article.
-
The transition state for coupled folding and binding of a disordered DNA binding domain resembles the unbound state.Nucleic Acids Res. 2024 Oct 28;52(19):11822-11837. doi: 10.1093/nar/gkae794. Nucleic Acids Res. 2024. PMID: 39315703 Free PMC article.
-
Membrane-Induced Folding of the Plant Stress Dehydrin Lti30.Plant Physiol. 2016 Jun;171(2):932-43. doi: 10.1104/pp.15.01531. Epub 2016 Apr 26. Plant Physiol. 2016. PMID: 27208263 Free PMC article.
References
-
- Ackerman, M.S. and Shortle, D. 2002. Robustness of the long-range structure in denatured staphylococcal nuclease to changes in amino acid sequence. Biochemistry 41: 13791–13797. - PubMed
-
- Bennett, M.C. 2005. The role of α-synuclein in neurodegenerative diseases. Pharmacol. Ther. 105: 311–331. - PubMed
-
- Bermel, W., Bertini, I., Felli, I.C., Lee, Y.M., Luchinat, C., and Pierattelli, R. 2006. Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J. Am. Chem. Soc. 128: 3918–3919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

