An inserted Gly residue fine tunes dynamics between mesophilic and thermophilic ribonucleases H
- PMID: 17088323
- PMCID: PMC2242442
- DOI: 10.1110/ps.062398606
An inserted Gly residue fine tunes dynamics between mesophilic and thermophilic ribonucleases H
Abstract
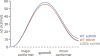
Dynamic processes are inherent properties of proteins and are crucial for a wide range of biological functions. To address how changes in protein sequence and structure affect dynamic processes, a quantitative comparison of microsecond-to-microsecond time scale conformational changes, measured by solution NMR spectroscopy, within homologous mesophilic and thermophilic ribonuclease H (RNase H) enzymes is presented. Kinetic transitions between the observed major state (high population) and alternate (low population) conformational state(s) of the substrate-binding handle region in RNase H from the mesophile Escherichia coli (ecRNH) and thermophile Thermus thermophilus (ttRNH) occur with similar kinetic exchange rate constants, but the difference in stability between exchanging conformers is smaller in ttRNH compared to ecRNH. The altered thermodynamic equilibrium between kinetically exchanging conformers in the thermophile is recapitulated in ecRNH by the insertion of a Gly residue within a putative hinge between alpha-helices B and C. This Gly insertion is conserved among thermophilic RNases H, and allows the formation of additional intrahelical hydrogen bonds. A Gly residue inserted between alpha-helices B and C appears to relieve unfavorable interactions in the transition state and alternate conformer(s) and represents an important adaptation to adjust conformational changes within RNase H for activity at high temperatures.
Figures





References
-
- Akke, M. 2002. NMR methods for characterizing microsecond to millisecond dynamics in recognition and catalysis. Curr. Opin. Struct. Biol. 12: 642–647. - PubMed
-
- Akke, M. and Palmer III, A.G. 1996. Monitoring macromolecular motions on microsecond to millisecond time scales by R 1ρ − R 1 constant relaxation time NMR spectroscopy. J. Am. Chem. Soc. 118: 911–912.
-
- Benkovic, S.J. and Hammes-Schiffer, S. 2003. A perspective on enzyme catalysis. Science 301: 1196–1202. - PubMed
-
- Butterwick, J.A., Loria, J.P., Astrof, N.S., Kroenke, C.D., Cole, R., Rance, M., and Palmer III, A.G. 2004. Multiple time scale backbone dynamics of homologous thermophilic and mesophilic ribonuclease HI enzymes. J. Mol. Biol. 339: 855–871. - PubMed
-
- Cavanagh, J., Fairbrother, W.J., Palmer III, A.G., and Skelton, N.J. 1996. Protein NMR spectroscopy: Principles and practice. Academic Press, San Diego, CA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

