Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants
- PMID: 17088344
- PMCID: PMC1828410
- DOI: 10.1128/IAI.01054-06
Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants
Abstract
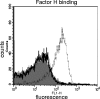
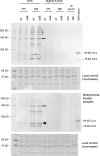
Nontypeable (NT) Haemophilus influenzae is an important cause of otitis media in children. We have shown previously that NT H. influenzae mutants defective in their ability to sialylate lipopolysaccharide (LPS), called siaB mutants, show attenuated virulence in a chinchilla model of experimental otitis media (EOM). We show that complement is a key arm of host innate immunity against NT H. influenzae-induced EOM. Depleting complement in chinchillas by use of cobra venom factor (CoVF) rendered two otherwise avirulent siaB mutants fully virulent and able to cause EOM with severity similar to that of wild-type strains. Clearance of infection caused by siaB mutants in CoVF-treated animals coincided with reappearance of C3. Wild-type strains were more resistant to direct complement-mediated killing than their siaB mutants. The serum-resistant strain bound less C3 and C4 than the serum-sensitive strain. Neither NT H. influenzae strain tested bound factor H (alternative complement pathway regulator). Selective activation of the alternative pathway resulted in more C3 binding to siaB mutants. LPS sialylation had a more profound impact on the amount of alternative-pathway-mediated C3 binding ( approximately 5-fold decrease in fluorescence) when LPS was the main C3 target, as occurred on the more serum-resistant strain. In contrast, only an approximately 1.5-fold decrease in fluorescence intensity of C3 binding was seen with the serum-sensitive strain, where surface proteins predominantly bound C3. Differences in binding sites for C3 and C4 may account for variations in serum resistance between NT H. influenzae strains, which in turn may impact their virulence. These data demonstrate a central role for complement in innate immune defenses against NT H. influenzae infections and specifically EOM.
Figures






Similar articles
-
Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media.Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8898-903. doi: 10.1073/pnas.1432026100. Epub 2003 Jul 10. Proc Natl Acad Sci U S A. 2003. PMID: 12855765 Free PMC article.
-
Role of complement in host defense against pneumococcal otitis media.Infect Immun. 2009 Mar;77(3):1121-7. doi: 10.1128/IAI.01148-08. Epub 2009 Jan 12. Infect Immun. 2009. PMID: 19139190 Free PMC article.
-
Modified lipooligosaccharide structure protects nontypeable Haemophilus influenzae from IgM-mediated complement killing in experimental otitis media.mBio. 2012 Jul 3;3(4):e00079-12. doi: 10.1128/mBio.00079-12. Print 2012. mBio. 2012. PMID: 22761391 Free PMC article.
-
Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines.Clin Infect Dis. 2008 Mar 1;46(5):726-31. doi: 10.1086/527396. Clin Infect Dis. 2008. PMID: 18230042 Review.
-
The role of complement in the host's defense against Haemophilus influenzae.J Infect Dis. 1992 Jun;165 Suppl 1:S62-5. doi: 10.1093/infdis/165-supplement_1-s62. J Infect Dis. 1992. PMID: 1588179 Review.
Cited by
-
Tracking insertion mutants within libraries by deep sequencing and a genome-wide screen for Haemophilus genes required in the lung.Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16422-7. doi: 10.1073/pnas.0906627106. Epub 2009 Sep 4. Proc Natl Acad Sci U S A. 2009. PMID: 19805314 Free PMC article.
-
Structure-Activity Relationship of Fluorinated Sialic Acid Inhibitors for Bacterial Sialylation.Bioconjug Chem. 2021 Jun 16;32(6):1047-1051. doi: 10.1021/acs.bioconjchem.1c00194. Epub 2021 May 27. Bioconjug Chem. 2021. PMID: 34043338 Free PMC article.
-
Panel 5: Microbiology and immunology panel.Otolaryngol Head Neck Surg. 2013 Apr;148(4 Suppl):E64-89. doi: 10.1177/0194599812459636. Otolaryngol Head Neck Surg. 2013. PMID: 23536533 Free PMC article. Review.
-
Genome-scale approaches to identify genes essential for Haemophilus influenzae pathogenesis.Front Cell Infect Microbiol. 2012 Mar 5;2:23. doi: 10.3389/fcimb.2012.00023. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919615 Free PMC article. Review.
-
Nontypable Haemophilus influenzae displays a prevalent surface structure molecular pattern in clinical isolates.PLoS One. 2011;6(6):e21133. doi: 10.1371/journal.pone.0021133. Epub 2011 Jun 16. PLoS One. 2011. PMID: 21698169 Free PMC article.
References
-
- Alper, C. A., and D. Balavitch. 1976. Cobra venom factor: evidence for its being altered cobra C3 (the third component of complement). Science 191:1275-1276. - PubMed
-
- Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

