A new method reveals microtubule minus ends throughout the meiotic spindle
- PMID: 17088423
- PMCID: PMC2064514
- DOI: 10.1083/jcb.200511112
A new method reveals microtubule minus ends throughout the meiotic spindle
Abstract
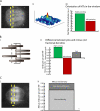
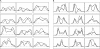
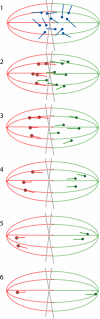
Anastral meiotic spindles are thought to be organized differently from astral mitotic spindles, but the field lacks the basic structural information required to describe and model them, including the location of microtubule-nucleating sites and minus ends. We measured the distributions of oriented microtubules in metaphase anastral spindles in Xenopus laevis extracts by fluorescence speckle microscopy and cross-correlation analysis. We localized plus ends by tubulin incorporation and combined this with the orientation data to infer the localization of minus ends. We found that minus ends are localized throughout the spindle, sparsely at the equator and at higher concentrations near the poles. Based on these data, we propose a model for maintenance of the metaphase steady-state that depends on continuous nucleation of microtubules near chromatin, followed by sorting and outward transport of stabilized minus ends, and, eventually, their loss near poles.
Figures
References
-
- Brinkley, B.R. 1985. Microtubule organizing centers. Annu. Rev. Cell Biol. 1:145–172. - PubMed
-
- Carazo-Salas, R.E., and E. Karsenti. 2003. Long-range communication between chromatin and microtubules in Xenopus egg extracts. Curr. Biol. 13:1728–1733. - PubMed
-
- Carazo-Salas, R.E., G. Guarguaglini, O.J. Gruss, A. Segref, E. Karsenti, and I.W. Mattaj. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 400:178–181. - PubMed
-
- Carazo-Salas, R.E., O.J. Gruss, I.W. Mattaj, and E. Karsenti. 2001. Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 3:228–234. - PubMed
-
- Desai, A., A. Murray, T.J. Mitchison, and C.E. Walczak. 1999. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61:385–412. - PubMed

