Reassembly of contractile actin cortex in cell blebs
- PMID: 17088428
- PMCID: PMC2064524
- DOI: 10.1083/jcb.200602085
Reassembly of contractile actin cortex in cell blebs
Abstract
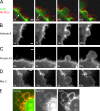
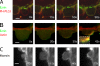
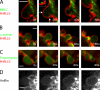
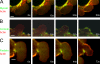
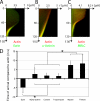
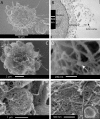
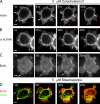
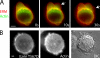
Contractile actin cortex is involved in cell morphogenesis, movement, and cytokinesis, but its organization and assembly are poorly understood. During blebbing, the membrane detaches from the cortex and inflates. As expansion ceases, contractile cortex re-assembles under the membrane and drives bleb retraction. This cycle enabled us to measure the temporal sequence of protein recruitment to the membrane during cortex reassembly and to explore dependency relationships. Expanding blebs were devoid of actin, but proteins of the erythrocytic submembranous cytoskeleton were present. When expansion ceased, ezrin was recruited to the membrane first, followed by actin, actin-bundling proteins, and, finally, contractile proteins. Complete assembly of the contractile cortex, which was organized into a cagelike mesh of filaments, took approximately 30 s. Cytochalasin D blocked recruitment of actin and alpha-actinin, but had no effect on membrane association of ankyrin B and ezrin. Ezrin played no role in actin nucleation, but was essential for tethering the membrane to the cortex. The Rho pathway was important for cortex assembly in blebs.
Figures
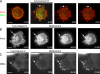
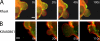
References
-
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. Watson. 2004. Molecular Biology of the Cell. 4th edition. Taylor and Francis, New York. 1463 pp.
-
- Barylko, B., D.D. Binns, and J.P. Albanesi. 2000. Regulation of the enzymatic and motor activities of myosin I. Biochim. Biophys. Acta. 1496:23–35. - PubMed
-
- Bennett, V., and A.J. Baines. 2001. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353–1392. - PubMed
-
- Bray, D., and J.G. White. 1988. Cortical flow in animal cells. Science. 239:883–888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

