Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation
- PMID: 17088433
- PMCID: PMC2118143
- DOI: 10.1084/jem.20061097
Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation
Abstract
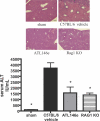
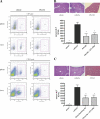
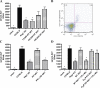
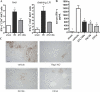
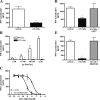
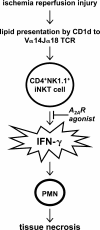
Ischemia reperfusion injury results from tissue damage during ischemia and ongoing inflammation and injury during reperfusion. Liver reperfusion injury is reduced by lymphocyte depletion or activation of adenosine A2A receptors (A2ARs) with the selective agonist 4-{3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]- prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester (ATL146e). We show that NKT cells are stimulated to produce interferon (IFN)-gamma by 2 h after the initiation of reperfusion, and the use of antibodies to deplete NK1.1-positive cells (NK and NKT) or to block CD1d-mediated glycolipid presentation to NKT cells replicates, but is not additive to, the protection afforded by ATL146e, as assessed by serum alanine aminotransferase elevation, histological necrosis, neutrophil accumulation, and serum IFN-gamma elevation. Reduced reperfusion injury observed in RAG-1 knockout (KO) mice is restored to the wild-type (WT) level by adoptive transfer of NKT cells purified from WT or A2AR KO mice but not IFN-gamma KO mice. Additionally, animals with transferred A2AR-/- NKT cells are not protected from hepatic reperfusion injury by ATL146e. In vitro, ATL146e potently inhibits both anti-CD3 and alpha-galactosylceramide-triggered production of IFN-gamma by NKT cells. These findings suggest that hepatic reperfusion injury is initiated by the CD1d-dependent activation of NKT cells, and the activation of these cells is inhibited by A2AR activation.
Figures
References
-
- Day, Y.J., M.A. Marshall, L. Huang, M.J. McDuffie, M.D. Okusa, and J. Linden. 2004. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G285–G293. - PubMed
-
- Cronstein, B.N. 1994. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 76:5–13. - PubMed
-
- Lappas, C.M., J.M. Rieger, and J. Linden. 2005. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 174:1073–1080. - PubMed
-
- Linden, J. 2001. Molecular approach to adenosine receptors: receptor- mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41:775–787. - PubMed
-
- Ohta, A., and M. Sitkovsky. 2001. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 414:916–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

