Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods
- PMID: 17088483
- PMCID: PMC1797701
- DOI: 10.1128/AAC.00957-06
Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods
Abstract
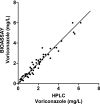
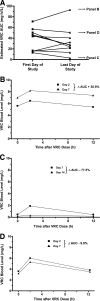
Voriconazole (VRC) is a broad-spectrum antifungal triazole with nonlinear pharmacokinetics. The utility of measurement of voriconazole blood levels for optimizing therapy is a matter of debate. Available high-performance liquid chromatography (HPLC) and bioassay methods are technically complex, time-consuming, or have a narrow analytical range. Objectives of the present study were to develop new, simple analytical methods and to assess variability of voriconazole blood levels in patients with invasive mycoses. Acetonitrile precipitation, reverse-phase separation, and UV detection were used for HPLC. A voriconazole-hypersusceptible Candida albicans mutant lacking multidrug efflux transporters (cdr1Delta/cdr1Delta, cdr2Delta/cdr2Delta, flu1Delta/flu1Delta, and mdr1Delta/mdr1Delta) and calcineurin subunit A (cnaDelta/cnaDelta) was used for bioassay. Mean intra-/interrun accuracies over the VRC concentration range from 0.25 to 16 mg/liter were 93.7% +/- 5.0%/96.5% +/- 2.4% (HPLC) and 94.9% +/- 6.1%/94.7% +/- 3.3% (bioassay). Mean intra-/interrun coefficients of variation were 5.2% +/- 1.5%/5.4% +/- 0.9% and 6.5% +/- 2.5%/4.0% +/- 1.6% for HPLC and bioassay, respectively. The coefficient of concordance between HPLC and bioassay was 0.96. Sequential measurements in 10 patients with invasive mycoses showed important inter- and intraindividual variations of estimated voriconazole area under the concentration-time curve (AUC): median, 43.9 mg x h/liter (range, 12.9 to 71.1) on the first and 27.4 mg x h/liter (range, 2.9 to 93.1) on the last day of therapy. During therapy, AUC decreased in five patients, increased in three, and remained unchanged in two. A toxic encephalopathy probably related to the increase of the VRC AUC (from 71.1 to 93.1 mg x h/liter) was observed. The VRC AUC decreased (from 12.9 to 2.9 mg x h/liter) in a patient with persistent signs of invasive aspergillosis. These preliminary observations suggest that voriconazole over- or underexposure resulting from variability of blood levels might have clinical implications. Simple HPLC and bioassay methods offer new tools for monitoring voriconazole therapy.
Figures
References
-
- Ally, R., D. Schürmann, W. Kreisel, G. Carosi, K. Aguirrebengoa, M. Hodges, P. Troke, A. Romero, et al. 2001. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin. Infect. Dis. 33:1447-1454. - PubMed
-
- Antoniadou, A., and D. P. Kontoyiannis. 2003. Status of combination therapy for refractory mycoses. Curr. Opin. Infect. Dis. 16:539-545. - PubMed
-
- Boyd, A. E., S. Modi, S. J. Howard, C. B. Moore, B. G. Keevil, and D. W. Denning. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241-1244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

