Plasma amprenavir pharmacokinetics and tolerability following administration of 1,400 milligrams of fosamprenavir once daily in combination with either 100 or 200 milligrams of ritonavir in healthy volunteers
- PMID: 17088488
- PMCID: PMC1797779
- DOI: 10.1128/AAC.00560-06
Plasma amprenavir pharmacokinetics and tolerability following administration of 1,400 milligrams of fosamprenavir once daily in combination with either 100 or 200 milligrams of ritonavir in healthy volunteers
Abstract
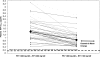
Once-daily (QD) fosamprenavir (FPV) at 1,400 mg boosted with low-dose ritonavir (RTV) at 200 mg is effective when it is used in combination regimens for the initial treatment of human immunodeficiency virus infection. Whether a lower RTV boosting dose (i.e., 100 mg QD) could ensure sufficient amprenavir (APV) concentrations with improved safety/tolerability is unknown. This randomized, two 14-day-period, crossover pharmacokinetic study compared the steady-state plasma APV concentrations, safety, and tolerability of FPV at 1,400 mg QD boosted with either 100 mg or 200 mg of RTV QD in 36 healthy volunteers. Geometric least-square (GLS) mean ratios and the associated 90% confidence intervals (CIs) were estimated for plasma APV maximum plasma concentrations (Cmax), the area under the plasma concentration-time curve over the dosing period (AUC0-tau), and trough concentrations (Ctau) during each dosing period. Equivalence between regimens (90% CIs of GLS mean ratios, 0.80 to 1.25) was observed for the plasma APV AUC0-tau (GLS mean ratio, 0.90 [90% CI, 0.84 to 0.96]) and Cmax (0.97 [90% CI, 0.91 to 1.04]). The APV Ctau was 38% lower with RTV at 100 mg QD than with RTV at 200 mg QD (GLS mean ratio, 0.62 [90% CI, 0.55 to 0.69]) but remained sixfold higher than the protein-corrected 50% inhibitory concentration for wild-type virus, with the lowest APV Ctau observed during the 100-mg QD period being nearly threefold higher. The GLS mean APV Ctau was 2.5 times higher than the historical Ctau for unboosted FPV at 1,400 mg twice daily. Fewer clinical adverse drug events and smaller increases in triglyceride levels were observed with the RTV 100-mg QD regimen. Clinical trials evaluating the efficacy and safety of FPV at 1,400 mg QD boosted by RTV at 100 mg QD are now under way with antiretroviral therapy-naïve patients.
Figures

References
-
- Cooper, C. L., R. P. van Heeswijk, K. Gallicano, and D. W. Cameron. 2003. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin. Infect. Dis. 36:1585-1592. - PubMed
-
- Dubé, M. P., D. Qian, H. Edmonson-Melançon, F. R. Sattler, D. Goodwin, C. Martinez, and V. Williams. 2002. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clin. Infect. Dis. 35:475-481. - PubMed
-
- Dubé, M. P., J. H. Stein, J. A. Aberg, C. J. Fichtenbaum, J. G. Gerber, K. T. Tashima, W. K. Henry, J. S. Currier, D. Sprecher, and M. J. Glesby for the Adult AIDS Clinical Trials Group Cardiovascular Subcommittee. 2003. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antretroviral therapy: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America and the Adult AIDS Clinical Trials Group. Clin. Infect. Dis. 37:613-627. - PubMed
-
- Gallant, J. E. 2004. Protease-inhibitor boosting in the treatment-experienced patient. AIDS Rev. 6:226-233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

