Wilms' tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA
- PMID: 17088532
- PMCID: PMC1634838
- DOI: 10.1073/pnas.0608357103
Wilms' tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA
Abstract
Wilms' tumor 1-associating protein (WTAP) has been reported to be a ubiquitously expressed nuclear protein. Although a relation to splicing factors has been postulated, its actual physiological function still remains to be elucidated. To investigate the role of WTAP, we generated WTAP-knockout mice and performed small interfering RNA (siRNA)-mediated knockdown analyses in primary cultured cells. In DNA microarrays using human umbilical vein endothelial cells, WTAP-targeted siRNA treatment resulted in markedly reduced expression of cell-cycle-related genes. siRNA-mediated WTAP knockdown down-regulated the stability of cyclin A2 mRNA through a nine-nucleotide essential sequence in cyclin A2 mRNA 3' UTR. WTAP knockdown induced G2 accumulation, which is partially rescued by adenoviral overexpression of cyclin A2. Moreover, WTAP-null mice exhibited proliferative failure with death resulting at approximately embryonic day 6.5, an etiology almost identical to cyclin A2-null mice. Collectively, these findings establish WTAP as an essential factor for the stabilization of cyclin A2 mRNA, thereby regulating G2/M cell-cycle transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
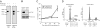
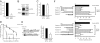
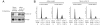

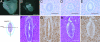
Similar articles
-
Wilms' tumor 1-associating protein regulates the proliferation of vascular smooth muscle cells.Circ Res. 2006 Dec 8;99(12):1338-46. doi: 10.1161/01.RES.0000252289.79841.d3. Epub 2006 Nov 9. Circ Res. 2006. PMID: 17095724
-
Wtap is required for differentiation of endoderm and mesoderm in the mouse embryo.Dev Dyn. 2008 Mar;237(3):618-29. doi: 10.1002/dvdy.21444. Dev Dyn. 2008. PMID: 18224709
-
Vascular biology and the sex of flies: regulation of vascular smooth muscle cell proliferation by wilms' tumor 1-associating protein.Trends Cardiovasc Med. 2007 Oct;17(7):230-4. doi: 10.1016/j.tcm.2007.08.002. Trends Cardiovasc Med. 2007. PMID: 17936204 Review.
-
Identification of Wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle.J Biol Chem. 2013 Nov 15;288(46):33292-302. doi: 10.1074/jbc.M113.500397. Epub 2013 Oct 7. J Biol Chem. 2013. PMID: 24100041 Free PMC article.
-
The A-type cyclins and the meiotic cell cycle in mammalian male germ cells.Int J Androl. 2004 Aug;27(4):192-9. doi: 10.1111/j.1365-2605.2004.00480.x. Int J Androl. 2004. PMID: 15271198 Review.
Cited by
-
The RNA m6A writer WTAP in diseases: structure, roles, and mechanisms.Cell Death Dis. 2022 Oct 7;13(10):852. doi: 10.1038/s41419-022-05268-9. Cell Death Dis. 2022. PMID: 36207306 Free PMC article. Review.
-
miR-139-5p Loss-Mediated WTAP Activation Contributes to Hepatocellular Carcinoma Progression by Promoting the Epithelial to Mesenchymal Transition.Front Oncol. 2021 Apr 15;11:611544. doi: 10.3389/fonc.2021.611544. eCollection 2021. Front Oncol. 2021. PMID: 33937023 Free PMC article.
-
N6 -Methyladenosine and Rheumatoid Arthritis: A Comprehensive Review.Front Immunol. 2021 Sep 24;12:731842. doi: 10.3389/fimmu.2021.731842. eCollection 2021. Front Immunol. 2021. PMID: 34630412 Free PMC article. Review.
-
Loss of WTAP Impairs Early Parthenogenetic Embryo Development.Animals (Basel). 2021 Jun 4;11(6):1675. doi: 10.3390/ani11061675. Animals (Basel). 2021. PMID: 34199793 Free PMC article.
-
Expression and roles of Wilms' tumor 1-associating protein in glioblastoma.Cancer Sci. 2012 Dec;103(12):2102-9. doi: 10.1111/cas.12022. Epub 2012 Oct 22. Cancer Sci. 2012. PMID: 22957919 Free PMC article.
References
-
- Little NA, Hastie ND, Davies RC. Hum Mol Genet. 2000;9:2231–2239. - PubMed
-
- Haber DA, Buckler AJ, Glaser T, Call KM, Pelletier J, Sohn RL, Douglass EC, Housman DE. Cell. 1990;61:1257–1269. - PubMed
-
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. Cell. 1993;74:679–691. - PubMed
-
- Hastie ND. Cell. 2001;106:391–394. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

