Self-renewal of embryonic stem cells by a small molecule
- PMID: 17088537
- PMCID: PMC1859921
- DOI: 10.1073/pnas.0608156103
Self-renewal of embryonic stem cells by a small molecule
Abstract
A cell-based screen of chemical libraries was carried out to identify small molecules that control the self-renewal of ES cells. A previously uncharacterized heterocycle, SC1, was discovered that allows one to propagate murine ES cells in an undifferentiated, pluripotent state under chemically defined conditions in the absence of feeder cells, serum, and leukemia inhibitory factor. Long-term SC1-expanded murine ES cells can be differentiated into cells of the three primary germ layers in vitro and also can generate chimeric mice and contribute to the germ line in vivo. Biochemical and cellular experiments suggest that SC1 works through dual inhibition of RasGAP and ERK1. Molecules of this kind may not only facilitate practical applications of stem cells in research and therapy, but also provide previously undescribed insights into the complex biology of stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
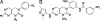

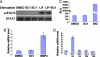


References
-
- Ying QL, Nichols J, Chambers I, Smith A. Cell. 2003;115:281–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

