Mullerian Inhibiting Substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer
- PMID: 17088539
- PMCID: PMC1859945
- DOI: 10.1073/pnas.0607959103
Mullerian Inhibiting Substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer
Abstract
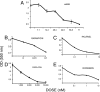
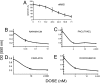
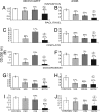
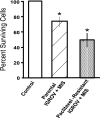
Mullerian Inhibiting Substance (MIS), a biological modifier that causes regression of Mullerian ducts in male embryos, is effective as a single agent in vitro and in vivo against human and mouse ovarian cancer cell lines expressing MIS type II receptor; however, little is known about how recombinant human MIS (rhMIS), now being scaled for preclinical trials, could be used in combination with cytotoxic or targeted chemotherapeutic agents. Mouse serous and endometrioid ovarian carcinoma cell lines were tested in vitro against rhMIS alone and with doxorubicin, paclitaxel, or cisplatin as agents in clinical use. Because MIS releases FK506 binding protein (FKBP12), which activates the mammalian target of rapamycin (mTOR) downstream of Akt, rhMIS and rapamycin combinations were tested. MIS increases p16 protein levels, and 5'-Aza-2'-deoxycytidine (AzadC) induces p16 mRNA; therefore, they were used in combination in vitro and in vivo with a human ovarian cancer cell line. A paclitaxel-resistant human ovarian cancer cell line and its parental line both respond to rhMIS in vitro. Additivity, synergy, or competition was observed with MIS and rapamycin, AzadC, doxorubicin, cisplatin, and paclitaxel, suggesting that MIS in combination with selective targeted therapies might achieve greater activity against ovarian cancer than the use of each individual agent alone. These assays and statistical analyses could be useful in selecting rhMIS and chemotherapeutic agent combinations that enhance clinical efficacy and reduce toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
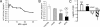
References
-
- Gustafson ML, Lee MM, Scully RE, Moncure AC, Hirakawa T, Goodman A, Muntz HG, Donahoe PK, MacLaughlin DT, Fuller AF., Jr N Engl J Med. 1992;326:466–471. - PubMed
-
- Lane AH, Lee MM, Fuller AF, Jr, Kehas DJ, Donahoe PK, MacLaughlin DT. Gynecol Oncol. 1999;73:51–55. - PubMed
-
- Lee MM, Donahoe PK, Silverman BL, Hasegawa T, Hasegawa Y, Gustafson ML, Chang YC, MacLaughlin DT. N Engl J Med. 1997;336:1480–1486. - PubMed
-
- Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. Cancer Res. 2003;63:1389–1397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

