An essential role of NF-kappaB in the "tumor-like" phenotype of arthritic synoviocytes
- PMID: 17088540
- PMCID: PMC1859946
- DOI: 10.1073/pnas.0607939103
An essential role of NF-kappaB in the "tumor-like" phenotype of arthritic synoviocytes
Abstract
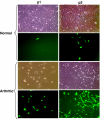
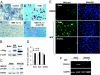
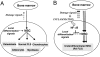
A hallmark of rheumatoid arthritis is the formation of an aggressive, tumor-like structure called pannus that erodes the joint. A major cellular component of the pannus is the fibroblast-like synoviocyte (FLS), whose morphology strikingly resembles that of a transformed cell, but underlying mechanisms of this "transformation" are not known. Here, using animal models of rheumatoid arthritis, we show that arthritic FLS contain a substantial (>30%) fraction of bone marrow-derived precursors that can differentiate in vitro into various mesenchymal cell types, but inflammation prevents the multilineage differentiation. We show that the transcription factor NF-kappaB plays the key role in the repression of osteogenic and adipogenic differentiation of arthritic FLS. Furthermore, we show that specific activation of NF-kappaB profoundly enhances proliferation, motility, and matrix-degrading activity of FLS. We thus propose that arthritic FLS are mesenchymal stem cells whose differentiation is arrested at early stages of differentiation by activation of NF-kappaB.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

