Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue
- PMID: 17088546
- PMCID: PMC1859949
- DOI: 10.1073/pnas.0604026103
Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue
Abstract
Lipoprotein lipase (LPL) has a central role in lipoprotein metabolism to maintain normal lipoprotein levels in blood and, through tissue specific regulation of its activity, to determine when and in what tissues triglycerides are unloaded. Recent data indicate that angiopoietin-like protein (Angptl)-4 inhibits LPL and retards lipoprotein catabolism. We demonstrate here that the N-terminal coiled-coil domain of Angptl-4 binds transiently to LPL and that the interaction results in conversion of the enzyme from catalytically active dimers to inactive, but still folded, monomers with decreased affinity for heparin. Inactivation occurred with less than equimolar ratios of Angptl-4 to LPL, was strongly temperature-dependent, and did not consume the Angptl-4. Furthermore, we show that Angptl-4 mRNA in rat adipose tissue turns over rapidly and that changes in the Angptl-4 mRNA abundance are inversely correlated to LPL activity, both during the fed-to-fasted and fasted-to-fed transitions. We conclude that Angptl-4 is a fasting-induced controller of LPL in adipose tissue, acting extracellularly on the native conformation in an unusual fashion, like an unfolding molecular chaperone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
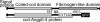
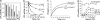
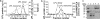

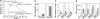

References
-
- Olivecrona T, Olivecrona G. In: Lipoproteins in Health and Disease. Betteridge DJ, Illingworth DR, Shepherd J, editors. London: Arnold; 1999. pp. 223–246.
-
- Preiss-Landl K, Zimmermann R, Hammerle G, Zechner R. Curr Opin Lipidol. 2002;13:471–481. - PubMed
-
- Merkel M, Eckel RH, Goldberg IJ. J Lipid Res. 2002;43:1997–2006. - PubMed
-
- Osborne JC, Jr, Bengtsson-Olivecrona G, Lee NS, Olivecrona T. Biochemistry. 1985;24:5606–5611. - PubMed
-
- Enerbäck S, Semb H, Tavernier J, Bjursell G, Olivecrona T. Gene. 1988;64:97–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

