A methyldiazene (HN=N-CH3)-derived species bound to the nitrogenase active-site FeMo cofactor: Implications for mechanism
- PMID: 17088552
- PMCID: PMC1693872
- DOI: 10.1073/pnas.0602130103
A methyldiazene (HN=N-CH3)-derived species bound to the nitrogenase active-site FeMo cofactor: Implications for mechanism
Abstract
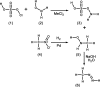
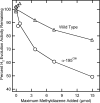
Methyldiazene (HN=N-CH3) isotopomers labeled with 15N at the terminal or internal nitrogens or with 13C or 2H were used as substrates for the nitrogenase alpha-195Gln-substituted MoFe protein. Freeze quenching under turnover traps an S = (1/2) state that has been characterized by EPR and 1H-, 15N-, and 13C-electron nuclear double resonance spectroscopies. These studies disclosed the following: (i) a methyldiazene-derived species is bound to the active-site FeMo cofactor; (ii) this species binds through an [-NHx] fragment whose N derives from the methyldiazene terminal N; and (iii) the internal N from methyldiazene probably does not bind to FeMo cofactor. These results constrain possible mechanisms for reduction of methyldiazene. In the Chatt-Schrock mechanism for N2 reduction, H atoms sequentially add to the distal N before N-N bond cleavage (d-mechanism). In a d-mechanism for methyldiazene reduction, a bound [-NHx] fragment only occurs after reduction by three electrons, which leads to N-N bond cleavage and the release of the first NH3. Thus, the appearance of bound [-NHx] is compatible with the d-mechanism only if it represents a late stage in the reduction process. In contrast are mechanisms where H atoms add alternately to distal and proximal nitrogens before N-N cleavage (a-mechanism) and release of the first NH3 after reduction by five electrons. An [-NHx] fragment would be bound at every stage of methyldiazene reduction in an a-mechanism. Although current information does not rule out the d-mechanism, the a-mechanism is more attractive because proton delivery to substrate has been specifically compromised in alpha-195Gln-substituted MoFe protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

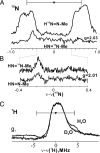
 N
N NH). For H15N
NH). For H15N N
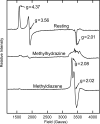
N CH3, ν = 34.808 GHz, g = 2.03, π/2 = 52 ns, τ = 400 ns, T = 25.352 μs, radio frequency (RF) = 20 μs, eight scans, 30 shots per point, 20-ms shot repetition time, 2 K. For HN
CH3, ν = 34.808 GHz, g = 2.03, π/2 = 52 ns, τ = 400 ns, T = 25.352 μs, radio frequency (RF) = 20 μs, eight scans, 30 shots per point, 20-ms shot repetition time, 2 K. For HN 15N
15N CH3, ν = 34.784 GHz, and other conditions are the same as for H15N
CH3, ν = 34.784 GHz, and other conditions are the same as for H15N N
N CH3. Spectra are normalized for comparison. (B) As in A, but narrower scan and g = 2.01, τ = 552 ns, and 15 scans. Backgrounds have been corrected by simple subtractions as needed. (C) Continuous wave (CW) 1H-ENDOR of m(CH3
CH3. Spectra are normalized for comparison. (B) As in A, but narrower scan and g = 2.01, τ = 552 ns, and 15 scans. Backgrounds have been corrected by simple subtractions as needed. (C) Continuous wave (CW) 1H-ENDOR of m(CH3 N
N NH) in H2O and D2O. Conditions were: microwave frequency of 35.057–35.171 GHz, modulation amplitude = 4 G, RF sweep speed = 1 MHz/s, bandwidth of RF broadened to 100 kHz, 2 K. Methyldiazene is abbreviated as HN
NH) in H2O and D2O. Conditions were: microwave frequency of 35.057–35.171 GHz, modulation amplitude = 4 G, RF sweep speed = 1 MHz/s, bandwidth of RF broadened to 100 kHz, 2 K. Methyldiazene is abbreviated as HN N
N Me.
Me.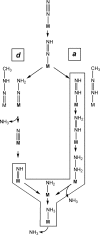
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

