No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block
- PMID: 17088870
- PMCID: PMC2014631
- DOI: 10.1038/sj.bjp.0706900
No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block
Abstract
Background & purpose: The therapeutically available quinolone antibiotic moxifloxacin has been used as a positive control for prolonging the QT interval in both clinical and non-clinical studies designed to assess the potential of new drugs to delay cardiac repolarization. Despite moxifloxacin prolonging QT, it has not been shown to cause torsades de pointes arrhythmias (TdP). Azithromycin is a macrolide antibiotic that has rarely been associated, clinically, with cases of proarrhythmia. As there is a lack of clinical data available, the cardiac safety of these drugs was assessed in a TdP-susceptible animal model by evaluating their repolarization and proarrhythmia effects.
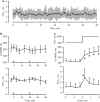
Experimental approach & key results: In transfected HEK cells, the IC(50)s for I (hERG) were 45+/-6 and 856+/-259 microg ml(-1) for moxifloxacin and azithromycin, respectively. Intravenous administration of 2 and 8 mg kg(-1) moxifloxacin (total peak-plasma concentrations 4.6+/-1.5 and 22.9+/-6.8 microg ml(-1)) prolonged the QT(c) in 6 anaesthetized dogs with chronic AV block by 7+/-3 and 21+/-19%, respectively. Similar intravenous doses of azithromycin (total peak-plasma concentrations 5.4+/-1.3 and 20.8+/-4.9 microg ml(-1)) had no electrophysiological effects in the same dogs. The reference compound, dofetilide (25 microg kg(-1) i.v.) caused QT(c) prolongation (29+/-15%) and TdP in all dogs. Beat-to-beat variability of repolarization (BVR), quantified as short-term variability of the left ventricular monophasic action potential duration, was only increased after dofetilide (1.8+/-0.7 to 3.8+/-1.5 ms; P<0.05).
Conclusion & implications: As neither moxifloxacin nor azithromycin caused TdP or an increase in the BVR, we conclude that both drugs can be used safely in clinical situations.
Figures





References
-
- Anderson ME, Mazur A, Yang T, Roden DM. Potassium current antagonist properties and proarrhythmic consequences of quinolone antibiotics. J Pharmacol Exp Ther. 2001;296:806–810. - PubMed
-
- Andriole VT, Haverstock DC, Choudhri SH. Retrospective analysis of the safety profile of oral moxifloxacin in elderly patients enrolled in clinical trials. Drug Safety. 2005;28:443–452. - PubMed
-
- Antoons G, Stengl M, Thomsen MB, Beekman JD, Vos MA, Sipido KR. Sarcoplasmic reticulum Ca2+ release and repolarization lability in myocytes from the dog with chronic atrioventricular block (cAVB) Biophys J. 2005;88:298A.
-
- Bischoff U, Schmidt C, Netzer R, Pongs O. Effects of fluoroquinolones on HERG currents. Eur J Pharmacol. 2000;406:341–343. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

