SPARC-induced migration of glioblastoma cell lines via uPA-uPAR signaling and activation of small GTPase RhoA
- PMID: 17088972
- PMCID: PMC1661847
SPARC-induced migration of glioblastoma cell lines via uPA-uPAR signaling and activation of small GTPase RhoA
Abstract
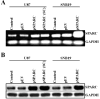
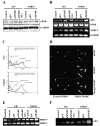
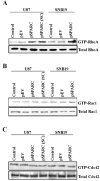
Secreted protein acidic and rich in cysteine (SPARC) is highly expressed in human gliomas where it promotes invasion and delays tumor growth, both in vitro and in vivo. SPARC, which interacts at the cell surface, has an impact on intracellular signaling and downstream gene expression changes, which might account for some of its effects on invasion and growth. Additionally in vitro studies demonstrated that SPARC delays growth, increases attachment, and modulates migration of tumor cells in an extracellular matrix-specific and concentration-dependent manner. Because the signaling aspect of this migration is neither well understood nor characterized, we overexpressed SPARC in both the minimally-invasive U87 cell line and in the most aggressive invasive cell line, SNB19. We first performed RT-PCR analysis and observed an upregulation of uPA and its receptor, uPAR. We also observed increased expression levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9). Western blot analysis confirmed these results, and the enzymatic activity of the metalloproteinases and uPA was further supported by zymography. Downstream of the uPA-uPAR interaction, upregulation of PI3-K occurred in cells overexpressing SPARC. Using GST-TRBD, we showed the upregulation of active GTP-bound RhoA, but neither Rac1 nor Cdc42 were activated. The inhibition of uPA and uPAR downregulated PI3-K activity and cell migration, as shown by matrigel invasion assay. A dorsal skin-fold chamber model revealed the high angiogenic activity of SPARC, though the proliferation of SPARC overexpressing cells was unaffected. Our results show that the small GTPase RhoA was a critical mediator of invasion or migration in the uPA-uPAR/PI3-K signaling pathway.
Figures

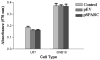
References
-
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. - PubMed
-
- Golembieski WA, Rempel SA. cDNA array analysis of SPARC-modulated changes in glioma gene expression. J Neurooncol. 2002;60:213–226. - PubMed
-
- Yamanaka M, Kanda K, Li NC, Fukumori T, Oka N, Kanayama HO, Kagawa S. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001;166:2495–2499. - PubMed
-
- Sakai N, Baba M, Nagasima Y, Kato Y, Hirai K, Kondo K, Kobayashi K, Yoshida M, Kaneko S, Kishida T, Kawakami S, Hosaka M, Inayama Y, Yao M. SPARC expression in primary human renal cell carcinoma: upregulation of SPARC in sarcomatoid renal carcinoma. Hum Pathol. 2001;32:1064–1070. - PubMed
-
- Paley PJ, Goff BA, Gown AM, Greer BE, Sage EH. Alterations in SPARC and VEGF immunoreactivity in epithelial ovarian cancer. Gynecol Oncol. 2000;78:336–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

