Rapid redistribution of synaptic PSD-95 in the neocortex in vivo
- PMID: 17090216
- PMCID: PMC1634879
- DOI: 10.1371/journal.pbio.0040370
Rapid redistribution of synaptic PSD-95 in the neocortex in vivo
Abstract
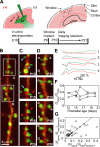
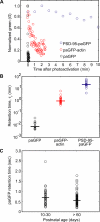
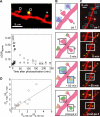
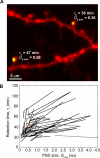
Most excitatory synapses terminate on dendritic spines. Spines vary in size, and their volumes are proportional to the area of the postsynaptic density (PSD) and synaptic strength. PSD-95 is an abundant multi-domain postsynaptic scaffolding protein that clusters glutamate receptors and organizes the associated signaling complexes. PSD-95 is thought to determine the size and strength of synapses. Although spines and their synapses can persist for months in vivo, PSD-95 and other PSD proteins have shorter half-lives in vitro, on the order of hours. To probe the mechanisms underlying synapse stability, we measured the dynamics of synaptic PSD-95 clusters in vivo. Using two-photon microscopy, we imaged PSD-95 tagged with GFP in layer 2/3 dendrites in the developing (postnatal day 10-21) barrel cortex. A subset of PSD-95 clusters was stable for days. Using two-photon photoactivation of PSD-95 tagged with photoactivatable GFP (paGFP), we measured the time over which PSD-95 molecules were retained in individual spines. Synaptic PSD-95 turned over rapidly (median retention times tau(r) is approximately 22-63 min from P10-P21) and exchanged with PSD-95 in neighboring spines by diffusion. PSDs therefore share a dynamic pool of PSD-95. Large PSDs in large spines captured more diffusing PSD-95 and also retained PSD-95 longer than small PSDs. Changes in the sizes of individual PSDs over days were associated with concomitant changes in PSD-95 retention times. Furthermore, retention times increased with developmental age (tau(r) is approximately 100 min at postnatal day 70) and decreased dramatically following sensory deprivation. Our data suggest that individual PSDs compete for PSD-95 and that the kinetic interactions between PSD molecules and PSDs are tuned to regulate PSD size.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures


Comment in
-
Kinetics of synaptic protein turnover regulate synaptic size.PLoS Biol. 2006 Nov;4(11):e404. doi: 10.1371/journal.pbio.0040404. Epub 2006 Nov 7. PLoS Biol. 2006. PMID: 20076499 Free PMC article. No abstract available.
References
-
- Beaulieu C, Colonnier M. A laminar analysis of the number of round-asymmetrical and flat-symmetrical synapses on spines, dendritic trunks, and cell bodies in area 17 of the cat. J Comp Neurol. 1985;231:180–189. - PubMed
-
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. - PubMed
-
- Wise SP, Fleshman JW, Jr, Jones EG. Maturation of pyramidal cell form in relation to developing afferent and efferent connections of rat somatic sensory cortex. Neuroscience. 1979;4:1275–1297. - PubMed
-
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. - PubMed
-
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol. 1996;373:340–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

