Comparative insights into questions of lepidopteran wing pattern homology
- PMID: 17090321
- PMCID: PMC1654149
- DOI: 10.1186/1471-213X-6-52
Comparative insights into questions of lepidopteran wing pattern homology
Abstract
Background: Butterfly and moth eyespots can share a similar appearance, involving multiple concentric rings of colored scales, but usually occurring in non-homologous positions on the wing. Within the butterflies, on the other hand, spots that share the same homologous position may not share the concentric ring structure; and, in butterfly species that have eyespots with concentric rings, ectopic eyespots with a similar ring structure can be induced by means of a simple epidermal wound. The extent to which all these eyespots, natural or induced, share similar genes and developmental mechanisms is investigated here by means of protein in-situ localizations in selected butterfly and moth species. In addition to looking at some of the transcription factors previously identified as being involved in eyespot formation, we also tested the involvement of candidate genes from the Wingless and TGF-beta signaling pathways as putative morphogens for eyespot development.
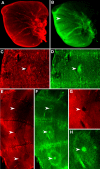
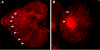
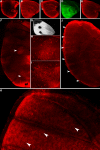
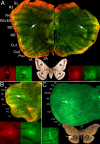
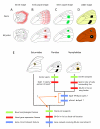
Results: Saturniid moth and nymphalid butterfly eyespots with concentric rings of color express at least two transcription factors, Distal-less and Engrailed, in the center of the future pattern. Nymphalid eyespots centers also express the ligand Wingless and an activated signal transducer, a phosphorylated Smad protein, but neither these proteins nor the previous two proteins are found in pierid spot centers, which consist of a single patch of color. Both butterfly wing patterns, however, express a third transcription factor, Spalt, a portion of whose expression domain maps to the black scales on the adult wing. Wounding a nymphalid wing, on the other hand, leads to upregulation of Distal-less, engrailed and spalt in subsets of cells around the wounding site, mimicking concentric eyespot development.
Conclusion: Wingless and TGF-beta ligands are both candidate morphogens involved in nymphalid butterfly eyespot formation. These eyespots, as well as saturniid moth eyespots with concentric circles, share two genes that are associated with the differentiation of the signaling cells in nymphalid eyespots. This commonality suggests that they may be produced via the same developmental mechanism despite their non-homologous location. By contrast, pierid butterfly spots of a single color share some of the same genes but appear to be produced by a different mechanism. Eyespots with concentric rings may have co-opted a wound healing genetic network during their evolution.
Figures



References
-
- Wagner GP. The biological homology concept. Ann Rev Ecol Syst. 1989;20:51–69. doi: 10.1146/annurev.es.20.110189.000411. - DOI
-
- Nijhout HF. The development and evolution of butterfly wing patterns. Washington, Smithsonian Institution Press; 1991. p. 297.
-
- Kristensen NP. Lepidoptera, moths and butterflies: Evolution, systematics and biogeography. In: Kristensen NP, editor. Handbook of Zoology. Volume 1. New York , Walter de Gruyter; 1999.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

