Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models
- PMID: 17090666
- PMCID: PMC1635021
- DOI: 10.1073/pnas.0608607103
Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models
Abstract
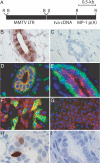
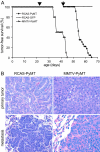
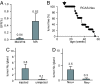
We have adapted the avian leukosis virus RCAS (replication-competent avian sarcoma-leukosis virus LTR splice acceptor)-mediated somatic gene transfer technique to introduce oncogenes into mammary cells in mice transgenic for the avian subgroup A receptor gene, tva, under control of the mouse mammary tumor virus (MMTV) promoter. Intraductal instillation of an RCAS vector carrying the polyoma middle T antigen (PyMT) gene (RCAS-PyMT) induced multiple, oligoclonal tumors within 3 weeks in infected mammary glands of MMTV-tva transgenic mice. The rapid appearance of these tumors from a relatively small pool of infected cells (estimated to be approximately 2 x 10(3) cells per gland by infection with RCAS carrying a GFP gene; RCAS-GFP) was accompanied by a high fraction of cells positive for Ki67, Cyclin D1, and c-Myc, implying strong proliferation competence. Furthermore, the tumors displayed greater cellular heterogeneity than did tumors arising in MMTV-PyMT mice, suggesting that RCAS-PyMT transforms a relatively immature cell type. Infection of mice transgenic for both MMTV-Wnt-1 and MMTV-tva with RCAS virus carrying an activated Neu oncogene dramatically enhanced tumor formation over what is observed in uninfected bitransgenic animals. We conclude that infection of mammary glands with retrovirus vectors is an efficient means to screen candidate oncogenes for their capacity to initiate or promote mammary carcinogenesis in the mouse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

