Barriers in phase I cancer clinical trials referrals and enrollment: five-year experience at the Princess Margaret Hospital
- PMID: 17092349
- PMCID: PMC1636658
- DOI: 10.1186/1471-2407-6-263
Barriers in phase I cancer clinical trials referrals and enrollment: five-year experience at the Princess Margaret Hospital
Abstract
Background: There is a paucity of literature on the referral outcome of patients seen in phase I trial clinics in academic oncology centres. This study aims to provide information on the accrual rate and to identify obstacles in the recruitment process.
Methods: A retrospective chart review was performed for all new patients referred and seen in the phase I clinic at the Princess Margaret Hospital between January 2000 and June 2005. Data on their demographics, medical history, and details of trial participation or non-entry were recorded.
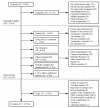
Results: A total of 667 new phase I referrals were seen during the stated period. Of these patients, 197 (29.5%) patients were enrolled into a phase I trial, and 64.5% of them started trial within 1 month of the initial visit. About a quarter (165 of 667) of the patients referred were deemed ineligible at their first visit, with the most frequent reasons for ineligibility being poor performance status, unacceptable bloodwork, too many prior treatments and rapid disease progression. The remaining 305 patients (45.7%) were potentially eligible at their initial visit, but never entered a phase I trial. The main reasons for their non-entry were patient refusal, other treatment recommended first, and lack of available trials or trial spots.
Conclusion: This study provides information on the clinical realities underlying a referral to a phase I clinic and eventual trial enrollment. Better selection of patients, appropriate education of referring physicians, and opening phase I trials with fewer restrictions on some criteria such as prior therapy may enhance their recruitment rates.
Figures
References
-
- Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, Siegler M. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol. 1995;13:1062–72. - PubMed
-
- Schutta KM, Burnett CB. Factors that influence a patient's decision to participate in a phase I cancer clinical trial. Oncol Nurs Forum. 2000;27:1435–8. - PubMed
-
- Yoder LH, O'Rourke TJ, Etnyre A, Spears DT, Brown TD. Expectations and experiences of patients with cancer participating in phase I clinical trials. Oncol Nurs Forum. 1997;24:891–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources