Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus
- PMID: 17092610
- PMCID: PMC3612500
- DOI: 10.1016/j.neurobiolaging.2006.09.015
Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus
Abstract
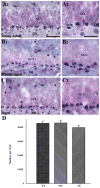
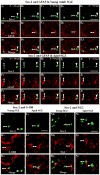
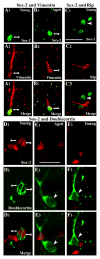
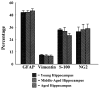
To investigate whether dramatically waned dentate neurogenesis during aging is linked to diminution in neural stem/progenitor cell (NSC) number, we counted cells immunopositive for Sox-2 (a putative marker of NSCs) in the subgranular zone (SGZ) of young, middle-aged and aged F344 rats. The young SGZ comprised approximately 50,000 Sox-2+ cells and this amount did not diminish with aging. Quantity of GFAP+ cells and vimentin+ radial glia also remained stable during aging in this region. Besides, in all age groups, analogous fractions of Sox-2+ cells expressed GFAP (astrocytes/NSCs), NG-2 (oligodendrocyte-progenitors/NSCs), vimentin (radial glia), S-100beta (astrocytes) and doublecortin (new neurons). Nevertheless, analyses of Sox-2+ cells with proliferative markers insinuated an increased quiescence of NSCs with aging. Moreover, the volume of rat-endothelial-cell-antigen-1+ capillaries (vascular-niches) within the SGZ exhibited an age-related decline, resulting in an increased expanse between NSCs and capillaries. Thus, decreased dentate neurogenesis during aging is not attributable to altered number or phenotype of NSCs. Instead, it appears to be an outcome of increased quiescence of NSCs due to changes in NSC milieu.
Conflict of interest statement
Figures
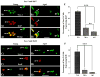


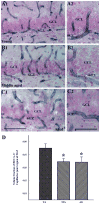

References
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;24:319–35. - PubMed
-
- Bernal GM, Peterson DA. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell. 2004;3:345–51. - PubMed
-
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–9. - PubMed
-
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

