The interplay of the N- and C-terminal domains of MCAK control microtubule depolymerization activity and spindle assembly
- PMID: 17093055
- PMCID: PMC1751331
- DOI: 10.1091/mbc.e06-08-0724
The interplay of the N- and C-terminal domains of MCAK control microtubule depolymerization activity and spindle assembly
Abstract
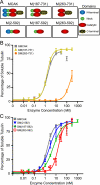
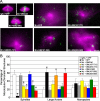
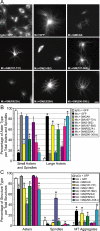
Spindle assembly and accurate chromosome segregation require the proper regulation of microtubule dynamics. MCAK, a Kinesin-13, catalytically depolymerizes microtubules, regulates physiological microtubule dynamics, and is the major catastrophe factor in egg extracts. Purified GFP-tagged MCAK domain mutants were assayed to address how the different MCAK domains contribute to in vitro microtubule depolymerization activity and physiological spindle assembly activity in egg extracts. Our biochemical results demonstrate that both the neck and the C-terminal domain are necessary for robust in vitro microtubule depolymerization activity. In particular, the neck is essential for microtubule end binding, and the C-terminal domain is essential for tight microtubule binding in the presence of excess tubulin heterodimer. Our physiological results illustrate that the N-terminal domain is essential for regulating microtubule dynamics, stimulating spindle bipolarity, and kinetochore targeting; whereas the C-terminal domain is necessary for robust microtubule depolymerization activity, limiting spindle bipolarity, and enhancing kinetochore targeting. Unexpectedly, robust MCAK microtubule (MT) depolymerization activity is not needed for sperm-induced spindle assembly. However, high activity is necessary for proper physiological MT dynamics as assayed by Ran-induced aster assembly. We propose that MCAK activity is spatially controlled by an interplay between the N- and C-terminal domains during spindle assembly.
Figures


Similar articles
-
Nup98 regulates bipolar spindle assembly through association with microtubules and opposition of MCAK.Mol Biol Cell. 2011 Mar 1;22(5):661-72. doi: 10.1091/mbc.E10-06-0478. Epub 2011 Jan 5. Mol Biol Cell. 2011. PMID: 21209315 Free PMC article.
-
Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation.Mol Biol Cell. 2004 Jun;15(6):2895-906. doi: 10.1091/mbc.e04-02-0082. Epub 2004 Apr 2. Mol Biol Cell. 2004. PMID: 15064354 Free PMC article.
-
The far C-terminus of MCAK regulates its conformation and spindle pole focusing.Mol Biol Cell. 2016 May 1;27(9):1451-64. doi: 10.1091/mbc.E15-10-0699. Epub 2016 Mar 3. Mol Biol Cell. 2016. PMID: 26941326 Free PMC article.
-
Mitotic centromere-associated kinesin (MCAK): a potential cancer drug target.Oncotarget. 2011 Dec;2(12):935-47. doi: 10.18632/oncotarget.416. Oncotarget. 2011. PMID: 22249213 Free PMC article. Review.
-
Kinesin-13s in mitosis: Key players in the spatial and temporal organization of spindle microtubules.Semin Cell Dev Biol. 2010 May;21(3):276-82. doi: 10.1016/j.semcdb.2010.01.016. Epub 2010 Jan 28. Semin Cell Dev Biol. 2010. PMID: 20109574 Free PMC article. Review.
Cited by
-
Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration.Dev Cell. 2012 Oct 16;23(4):716-28. doi: 10.1016/j.devcel.2012.08.010. Epub 2012 Sep 20. Dev Cell. 2012. PMID: 23000142 Free PMC article.
-
PLK1 phosphorylates mitotic centromere-associated kinesin and promotes its depolymerase activity.J Biol Chem. 2011 Jan 28;286(4):3033-46. doi: 10.1074/jbc.M110.165340. Epub 2010 Nov 15. J Biol Chem. 2011. PMID: 21078677 Free PMC article.
-
Aurora A orchestrates entosis by regulating a dynamic MCAK-TIP150 interaction.J Mol Cell Biol. 2014 Jun;6(3):240-54. doi: 10.1093/jmcb/mju016. Epub 2014 May 20. J Mol Cell Biol. 2014. PMID: 24847103 Free PMC article.
-
KIF2C is essential for meiosis and manchette dynamics in male mice.Front Cell Dev Biol. 2025 Mar 27;13:1523593. doi: 10.3389/fcell.2025.1523593. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40213390 Free PMC article.
-
How kinesin motor proteins drive mitotic spindle function: Lessons from molecular assays.Semin Cell Dev Biol. 2010 May;21(3):260-8. doi: 10.1016/j.semcdb.2010.01.018. Epub 2010 Jan 28. Semin Cell Dev Biol. 2010. PMID: 20109570 Free PMC article. Review.
References
-
- Adio S., Reth J., Bathe F., Woehlke G. Review: regulation mechanisms of Kinesin-1. J. Muscle Res. Cell Motil. 2006;27:153–160. - PubMed
-
- Andersen S. S., Ashford A. J., Tournebize R., Gavet O., Sobel A., Hyman A. A., Karsenti E. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 1997;389:640–643. - PubMed
-
- Andrews P. D., Ovechkina Y., Morrice N., Wagenbach M., Duncan K., Wordeman L., Swedlow J. R. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. - PubMed
-
- Banks J. D., Heald R. Adenomatous polyposis coli associates with the microtubule-destabilizing protein XMCAK. Curr. Biol. 2004;14:2033–2038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

