PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155
- PMID: 17093057
- PMCID: PMC1751330
- DOI: 10.1091/mbc.e06-06-0570
PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155
Abstract
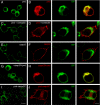
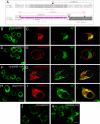
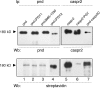
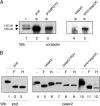
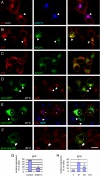
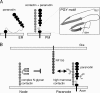
Formation of nodes of Ranvier requires contact of axons with myelinating glial cells, generating specialized axo-glial subdomains. Caspr/paranodin is required for the formation of septate-like junctions at paranodes, whereas the related caspr2 is essential for the organization of juxtaparanodes. The molecular mechanisms underlying the segregation of these related glycoproteins within distinct complexes are poorly understood. Exit of paranodin from the endoplasmic reticulum (ER) is mediated by its interaction with F3/contactin. Using domain swapping with caspr2, we mapped a motif with Pro-Gly-Tyr repeats (PGY) in the ectodomain of paranodin responsible for its ER retention. Deletion of PGY allows cell surface delivery of paranodin bypassing the calnexin-calreticulin quality control. Conversely, insertion of PGY in caspr2 or NrCAM blocks these proteins in the ER. PGY is a novel type of processing signal that compels chaperoning of paranodin by contactin. Contactin associated with paranodin is expressed at the cell surface with high-mannose N-glycans. Using mutant CHO lines altered in the processing of N-linked carbohydrates, we show that the high-mannose glycoform of contactin strongly binds neurofascin-155, its glial partner at paranodes. Thus, the unconventional processing of paranodin and contactin may determine the selective association of axo-glial complexes at paranodes.
Figures



References
-
- Baldwin T. A., Ostergaard H. L. The protein-tyrosine phosphatase CD45 reaches the cell surface via golgi-dependent and -independent pathways. J. Biol. Chem. 2002;277:50333–50340. - PubMed
-
- Bhat M. A., et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. - PubMed
-
- Bichet D., Cornet V., Geib S., Carlier E., Volsen S., Hoshi T., Mori Y., De Waard M. The I-II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 2000;25:177–190. - PubMed
-
- Bonnon C., Goutebroze L., Denisenko-Nehrbass N., Girault J. A., Faivre-Sarrailh C. The paranodal complex of F3/contactin and caspr/paranodin traffics to the cell surface via a non-conventional pathway. J. Biol. Chem. 2003;278:48339–48347. - PubMed
-
- Boyle M. E., Berglund E. O., Murai K. K., Weber L., Peles E., Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

