Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells
- PMID: 17093058
- PMCID: PMC1751327
- DOI: 10.1091/mbc.e06-05-0471
Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells
Abstract
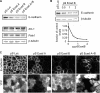
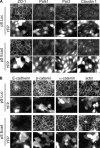
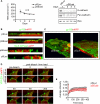
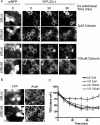
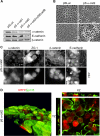
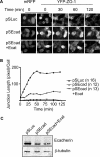
E-cadherin forms calcium-dependent homophilic intercellular adhesions between epithelial cells. These contacts regulate multiple aspects of cell behavior, including the organization of intercellular tight junctions (TJs). To distinguish between the roles of E-cadherin in formation versus maintenance of junctions, Madin-Darby canine kidney (MDCK) cells were depleted of E-cadherin by RNA interference. Surprisingly, reducing E-cadherin expression had little effect on the protein levels or localization of adherens junction (AJ) or TJ markers. The cells underwent morphological changes, as the normally flat apical surface swelled into a dome. However, apical-basal polarity was not compromised, transmembrane resistance was normal, and zonula occludin protein 1 dynamics at the TJs were unchanged. Additionally, an E-cadherin/Cadherin-6 double knockdown also failed to disrupt established TJs, although beta-catenin was lost from the cell cortex. Nevertheless, cells depleted of E-cadherin failed to properly reestablish cell polarity after junction disassembly. Recovery of cell-cell adhesion, transepithelial resistance, and the localization of TJ and AJ markers were all delayed. In contrast, depletion of alpha-catenin caused long-term disruption of junctions. These results indicate that E-cadherin and Cadherin-6 function as a scaffold for the construction of polarized structures, and they become largely dispensable in mature junctions, whereas alpha-catenin is essential for the maintenance of functional junctions.
Figures



References
-
- Adams C. L., Nelson W. J. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 1998;10:572–577. - PubMed
-
- Ando-Akatsuka Y., Yonemura S., Itoh M., Furuse M., Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell Physiol. 1999;179:115–125. - PubMed
-
- Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. - PubMed
-
- Boggon T. J., Murray J., Chappuis-Flament S., Wong E., Gumbiner B. M., Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

