FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX
- PMID: 17093062
- PMCID: PMC1751318
- DOI: 10.1091/mbc.e06-03-0207
FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX
Abstract
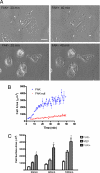
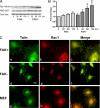
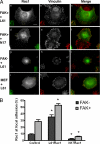
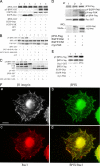
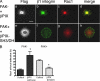
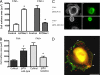
FAK, a cytoplasmic protein tyrosine kinase, is activated and localized to focal adhesions upon cell attachment to extracellular matrix. FAK null cells spread poorly and exhibit altered focal adhesion turnover. Rac1 is a member of the Rho-family GTPases that promotes membrane ruffling, leading edge extension, and cell spreading. We investigated the activation and subcellular location of Rac1 in FAK null and FAK reexpressing fibroblasts. FAK reexpressers had a more robust pattern of Rac1 activation after cell adhesion to fibronectin than the FAK null cells. Translocation of Rac1 to focal adhesions was observed in FAK reexpressers, but seldom in FAK null cells. Experiments with constitutively active L61Rac1 and dominant negative N17Rac1 indicated that the activation state of Rac1 regulated its localization to focal adhesions. We demonstrated that FAK tyrosine-phosphorylated betaPIX and thereby increased its binding to Rac1. In addition, betaPIX facilitated the targeting of activated Rac1 to focal adhesions and the efficiency of cell spreading. These data indicate that FAK has a role in the activation and focal adhesion translocation of Rac1 through the tyrosine phosphorylation of betaPIX.
Figures
Similar articles
-
Polyamine-dependent activation of Rac1 is stimulated by focal adhesion-mediated Tiam1 activation.Cell Adh Migr. 2010 Jul-Sep;4(3):419-30. doi: 10.4161/cam.4.3.12043. Epub 2010 Jul 14. Cell Adh Migr. 2010. PMID: 20448461 Free PMC article.
-
p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase.Exp Cell Res. 2005 Jul 15;307(2):315-28. doi: 10.1016/j.yexcr.2005.03.028. Exp Cell Res. 2005. PMID: 15893751
-
Invasion of host cells by Salmonella typhimurium requires focal adhesion kinase and p130Cas.Mol Biol Cell. 2006 Nov;17(11):4698-708. doi: 10.1091/mbc.e06-06-0492. Epub 2006 Aug 16. Mol Biol Cell. 2006. PMID: 16914515 Free PMC article.
-
AlphaPIX and betaPIX and their role in focal adhesion formation.Eur J Cell Biol. 2006 Apr;85(3-4):265-74. doi: 10.1016/j.ejcb.2005.10.007. Epub 2005 Dec 5. Eur J Cell Biol. 2006. PMID: 16337026 Review.
-
Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility.Curr Opin Cell Biol. 2009 Oct;21(5):676-83. doi: 10.1016/j.ceb.2009.05.006. Epub 2009 Jun 12. Curr Opin Cell Biol. 2009. PMID: 19525103 Free PMC article. Review.
Cited by
-
Smooth muscle hyperplasia due to loss of smooth muscle α-actin is driven by activation of focal adhesion kinase, altered p53 localization and increased levels of platelet-derived growth factor receptor-β.Hum Mol Genet. 2013 Aug 1;22(15):3123-37. doi: 10.1093/hmg/ddt167. Epub 2013 Apr 15. Hum Mol Genet. 2013. PMID: 23591991 Free PMC article.
-
Wnt5b integrates Fak1a to mediate gastrulation cell movements via Rac1 and Cdc42.Open Biol. 2020 Feb;10(2):190273. doi: 10.1098/rsob.190273. Epub 2020 Feb 26. Open Biol. 2020. PMID: 32097584 Free PMC article.
-
Angiogenesis within atherosclerotic plaques: Mechanical regulation, molecular mechanism and clinical diagnosis.Mechanobiol Med. 2025 Feb 1;3(1):100114. doi: 10.1016/j.mbm.2025.100114. eCollection 2025 Mar. Mechanobiol Med. 2025. PMID: 40396135 Free PMC article. Review.
-
Factor XII signaling via uPAR-integrin β1 axis promotes tubular senescence in diabetic kidney disease.Nat Commun. 2024 Sep 11;15(1):7963. doi: 10.1038/s41467-024-52214-8. Nat Commun. 2024. PMID: 39261453 Free PMC article.
-
FHL2 mediates podocyte Rac1 activation and foot process effacement in hypertensive nephropathy.Sci Rep. 2019 Apr 30;9(1):6693. doi: 10.1038/s41598-019-42328-1. Sci Rep. 2019. PMID: 31040292 Free PMC article.
References
-
- Arthur W. T., Petch L. A., Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 2000;10:719–722. - PubMed
-
- Benard V., Bohl B. P., Bokoch G. M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

