Myosin light chain kinase is not a regulator of synaptic vesicle trafficking during repetitive exocytosis in cultured hippocampal neurons
- PMID: 17093082
- PMCID: PMC6674773
- DOI: 10.1523/JNEUROSCI.3400-06.2006
Myosin light chain kinase is not a regulator of synaptic vesicle trafficking during repetitive exocytosis in cultured hippocampal neurons
Abstract
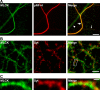
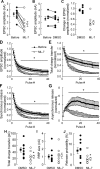
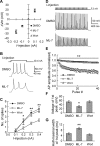
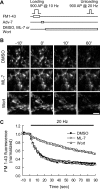
The mechanism by which synaptic vesicles (SVs) are recruited to the release site is poorly understood. One candidate mechanism for trafficking of SVs is the myosin-actin motor system. Myosin activity is modulated by myosin light chain kinase (MLCK), which in turn is activated by calmodulin. Ca(2+) signaling in presynaptic terminals, therefore, may serve to regulate SV mobility along actin filaments via MLCK. Previous studies in different types of synapses have supported such a hypothesis. Here, we further investigated the role of MLCK in neurotransmitter release at glutamatergic synapses in cultured hippocampal neurons by examining the effects of two MLCK inhibitors, 1-(5-iodonaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine.HCl (ML-7) and wortmannin. Bath application of ML-7 enhanced short-term depression of EPSCs to repetitive stimulation, whereas it reduced presynaptic release probability. However, ML-7 also inhibited action potential amplitude and voltage-gated Ca(2+) channel currents. These effects were not mimicked by wortmannin, suggesting that ML-7 was not specific to MLCK in hippocampal neurons. When SV exocytosis was directly triggered by a Ca(2+) ionophore, calcimycin, to bypass voltage-gated Ca(2+) channels, ML-7 had no effect on neurotransmitter release. Furthermore, when SV exocytosis elicited by electrical field stimulation was monitored by styryl dye, FM1-43 [N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl)pyridinium dibromide], the unloading kinetics of the dye was not altered in the presence of wortmannin. These data indicate that MLCK is not a major regulator of presynaptic SV trafficking during repetitive exocytosis at hippocampal synapses.
Figures


Similar articles
-
Myosin light chain kinase facilitates endocytosis of synaptic vesicles at hippocampal boutons.J Neurochem. 2016 Jul;138(1):60-73. doi: 10.1111/jnc.13635. Epub 2016 May 16. J Neurochem. 2016. PMID: 27062289 Free PMC article.
-
Potassium ion- and nitric oxide-induced exocytosis from populations of hippocampal synapses during synaptic maturation in vitro.Neuroscience. 1997 Oct;80(4):1057-73. doi: 10.1016/s0306-4522(97)00152-8. Neuroscience. 1997. PMID: 9284060
-
Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity.J Neurosci. 2006 Nov 8;26(45):11670-81. doi: 10.1523/JNEUROSCI.3321-06.2006. J Neurosci. 2006. PMID: 17093089 Free PMC article.
-
Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction.Neuroscientist. 2005 Apr;11(2):138-47. doi: 10.1177/1073858404271679. Neuroscientist. 2005. PMID: 15746382 Review.
-
The synaptic vesicle cycle revisited.Neuron. 2000 Nov;28(2):317-20. doi: 10.1016/s0896-6273(00)00109-4. Neuron. 2000. PMID: 11144340 Review. No abstract available.
Cited by
-
Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin.J Neurosci. 2007 Dec 12;27(50):13691-700. doi: 10.1523/JNEUROSCI.3910-07.2007. J Neurosci. 2007. PMID: 18077680 Free PMC article.
-
Calmodulin Bidirectionally Regulates Evoked and Spontaneous Neurotransmitter Release at Retinal Ribbon Synapses.eNeuro. 2021 Jan 6;8(1):ENEURO.0257-20.2020. doi: 10.1523/ENEURO.0257-20.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33293457 Free PMC article.
-
Myosin accelerates synaptic vesicle recycling in the motor nerve endings.Dokl Biol Sci. 2016 Sep;470(1):217-219. doi: 10.1134/S0012496616050112. Epub 2016 Nov 8. Dokl Biol Sci. 2016. PMID: 27822755
-
Myosin light chain kinase accelerates vesicle endocytosis at the calyx of Held synapse.J Neurosci. 2014 Jan 1;34(1):295-304. doi: 10.1523/JNEUROSCI.3744-13.2014. J Neurosci. 2014. PMID: 24381290 Free PMC article.
-
Fos and Jun potentiate individual release sites and mobilize the reserve synaptic vesicle pool at the Drosophila larval motor synapse.Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):4000-5. doi: 10.1073/pnas.0806064106. Epub 2009 Feb 19. Proc Natl Acad Sci U S A. 2009. PMID: 19228945 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
