Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity
- PMID: 17093089
- PMCID: PMC6674776
- DOI: 10.1523/JNEUROSCI.3321-06.2006
Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity
Abstract
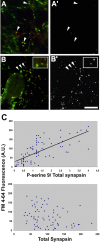
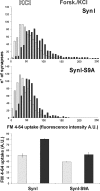
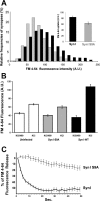
Protein kinase A (PKA) modulates several steps of synaptic transmission. However, the identification of the mediators of these effects is as yet incomplete. Synapsins are synaptic vesicle (SV)-associated phosphoproteins that represent the major presynaptic targets of PKA. We show that, in hippocampal neurons, cAMP-dependent pathways affect SV exocytosis and that this effect is primarily brought about through synapsin I phosphorylation. Phosphorylation by PKA, by promoting dissociation of synapsin I from SVs, enhances the rate of SV exocytosis on stimulation. This effect becomes relevant when neurons are challenged with sustained stimulation, because it appears to counteract synaptic depression and accelerate recovery from depression by fostering the supply of SVs from the reserve pool to the readily releasable pool. In contrast, synapsin phosphorylation appears to be dispensable for the effects of cAMP on the frequency and amplitude of spontaneous synaptic currents and on the amplitude of evoked synaptic currents. The modulation of depolarization-evoked SV exocytosis by PKA phosphorylation of synapsin I is primarily caused by calmodulin (CaM)-dependent activation of cAMP pathways rather than by direct activation of CaM kinases. These data define a hierarchical crosstalk between cAMP- and CaM-dependent cascades and point to synapsin as a major effector of PKA in the modulation of activity-dependent SV exocytosis.
Figures







Similar articles
-
Inhibition of exocytosis or endocytosis blocks activity-dependent redistribution of synapsin.J Neurochem. 2012 Jan;120(2):248-58. doi: 10.1111/j.1471-4159.2011.07579.x. Epub 2011 Dec 2. J Neurochem. 2012. PMID: 22066784
-
Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles.J Neurosci. 2008 Oct 22;28(43):10835-43. doi: 10.1523/JNEUROSCI.0924-08.2008. J Neurosci. 2008. PMID: 18945891 Free PMC article.
-
Myosin light chain kinase is not a regulator of synaptic vesicle trafficking during repetitive exocytosis in cultured hippocampal neurons.J Neurosci. 2006 Nov 8;26(45):11606-14. doi: 10.1523/JNEUROSCI.3400-06.2006. J Neurosci. 2006. PMID: 17093082 Free PMC article.
-
How synapsin I may cluster synaptic vesicles.Semin Cell Dev Biol. 2011 Jun;22(4):393-9. doi: 10.1016/j.semcdb.2011.07.006. Epub 2011 Jul 21. Semin Cell Dev Biol. 2011. PMID: 21798362 Review.
-
The synapsins: key actors of synapse function and plasticity.Prog Neurobiol. 2010 Aug;91(4):313-48. doi: 10.1016/j.pneurobio.2010.04.006. Epub 2010 May 10. Prog Neurobiol. 2010. PMID: 20438797 Review.
Cited by
-
Ovarian Function Modulates the Effects of Long-Chain Polyunsaturated Fatty Acids on the Mouse Cerebral Cortex.Front Cell Neurosci. 2018 Apr 24;12:103. doi: 10.3389/fncel.2018.00103. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29740285 Free PMC article.
-
NMDA-type glutamate receptors participate in reduction of food intake following hindbrain melanocortin receptor activation.Am J Physiol Regul Integr Comp Physiol. 2015 Jan 1;308(1):R1-9. doi: 10.1152/ajpregu.00388.2014. Epub 2014 Nov 12. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25394828 Free PMC article.
-
Regulation of Neurotransmitter Release by Amyloid Precursor Protein Through Synapsin Phosphorylation.Neurochem Res. 2019 Mar;44(3):683-691. doi: 10.1007/s11064-017-2418-2. Epub 2017 Oct 19. Neurochem Res. 2019. PMID: 29052089
-
A specific role for Ca2+-dependent adenylyl cyclases in recovery from adaptive presynaptic silencing.J Neurosci. 2008 May 14;28(20):5159-68. doi: 10.1523/JNEUROSCI.5317-07.2008. J Neurosci. 2008. PMID: 18480272 Free PMC article.
-
Phosphorylation of synapsin I by cyclin-dependent kinase-5 sets the ratio between the resting and recycling pools of synaptic vesicles at hippocampal synapses.J Neurosci. 2014 May 21;34(21):7266-80. doi: 10.1523/JNEUROSCI.3973-13.2014. J Neurosci. 2014. PMID: 24849359 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
