Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells
- PMID: 17093090
- PMCID: PMC6674781
- DOI: 10.1523/JNEUROSCI.3656-06.2006
Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells
Abstract
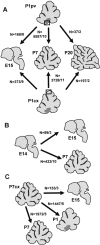
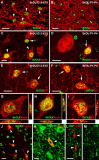
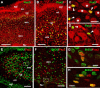
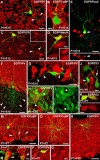
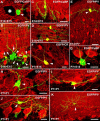
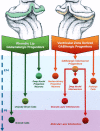
Different cerebellar phenotypes are generated according to a precise spatiotemporal schedule, in which projection neurons precede local interneurons. Glutamatergic neurons develop from the rhombic lip, whereas GABAergic neurons originate from the ventricular neuroepithelium. Progenitors in these germinal layers are committed toward specific phenotypes already at early ontogenetic stages. GABAergic interneurons are thought to derive from a subset of ventricular zone cells, which migrate in the white matter and proliferate up to postnatal life. During this period, different interneuron categories are produced according to an inside-out sequence, from the deep nuclei to the molecular layer (we show here that nuclear interneurons are also born during late embryonic and early postnatal days, after glutamatergic and GABAergic projection neurons). To ask whether distinct interneuron phenotypes share common precursors or derive from multiple fate-restricted progenitors, we examined the behavior of embryonic and postnatal rat cerebellar cells heterotopically/heterochronically transplanted to syngenic hosts. In all conditions, donor cells achieved a high degree of integration in the cerebellar cortex and deep nuclei and acquired GABAergic interneuron phenotypes appropriate for the host age and engraftment site. Therefore, contrary to other cerebellar types, which derive from dedicated precursors, GABAergic interneurons are produced by a common pool of progenitors, which maintain their full developmental potentialities up to late ontogenetic stages and adopt mature identities in response to local instructive cues. In this way, the numbers and types of inhibitory interneurons can be set by spatiotemporally patterned signals to match the functional requirements of developing cerebellar circuits.
Figures



Similar articles
-
Laminar fate and phenotype specification of cerebellar GABAergic interneurons.J Neurosci. 2009 May 27;29(21):7079-91. doi: 10.1523/JNEUROSCI.0957-09.2009. J Neurosci. 2009. PMID: 19474334 Free PMC article.
-
Specification and differentiation of cerebellar GABAergic neurons.Cerebellum. 2012 Jun;11(2):434-5. doi: 10.1007/s12311-011-0324-8. Cerebellum. 2012. PMID: 22090364 Review.
-
Extracerebellar progenitors grafted to the neurogenic milieu of the postnatal rat cerebellum adapt to the host environment but fail to acquire cerebellar identities.Eur J Neurosci. 2010 Apr;31(8):1340-51. doi: 10.1111/j.1460-9568.2010.07167.x. Epub 2010 Apr 9. Eur J Neurosci. 2010. PMID: 20384777
-
Heterogeneity and Bipotency of Astroglial-Like Cerebellar Progenitors along the Interneuron and Glial Lineages.J Neurosci. 2015 May 13;35(19):7388-402. doi: 10.1523/JNEUROSCI.5255-14.2015. J Neurosci. 2015. PMID: 25972168 Free PMC article.
-
Development of cerebellar GABAergic interneurons: origin and shaping of the "minibrain" local connections.Cerebellum. 2008;7(4):523-9. doi: 10.1007/s12311-008-0079-z. Cerebellum. 2008. PMID: 19002744 Review.
Cited by
-
Cerebellum Lecture: the Cerebellar Nuclei-Core of the Cerebellum.Cerebellum. 2024 Apr;23(2):620-677. doi: 10.1007/s12311-022-01506-0. Epub 2023 Feb 13. Cerebellum. 2024. PMID: 36781689 Free PMC article. Review.
-
The Role of PAX2 in Neurodevelopment and Disease.Neuropsychiatr Dis Treat. 2021 Dec 7;17:3559-3567. doi: 10.2147/NDT.S332747. eCollection 2021. Neuropsychiatr Dis Treat. 2021. PMID: 34908837 Free PMC article. Review.
-
Zfp423/ZNF423 regulates cell cycle progression, the mode of cell division and the DNA-damage response in Purkinje neuron progenitors.Development. 2017 Oct 15;144(20):3686-3697. doi: 10.1242/dev.155077. Epub 2017 Sep 11. Development. 2017. PMID: 28893945 Free PMC article.
-
Olig3 regulates early cerebellar development.Elife. 2021 Feb 16;10:e64684. doi: 10.7554/eLife.64684. Elife. 2021. PMID: 33591268 Free PMC article.
-
An aberrant cerebellar development in mice lacking matrix metalloproteinase-3.Mol Neurobiol. 2012 Feb;45(1):17-29. doi: 10.1007/s12035-011-8215-z. Epub 2011 Nov 23. Mol Neurobiol. 2012. PMID: 22108898
References
-
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. - PubMed
-
- Altman J, Bayer SA. Prenatal development of the cerebellar system in the rat. I. Cytogenesis and histogenesis of the deep nuclei and the cortex of the cerebellum. J Comp Neurol. 1978;179:23–48. - PubMed
-
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structures and functions. New York: CRC; 1997.
-
- Baader S, Bergmann M, Mertz K, Fox PA, Gerdes J, Oberdick J, Schilling K. The differentiation of cerebellar interneurons is independent of their mitotic history. Neuroscience. 1999;90:1243–1254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
