Glomerulus-specific, long-latency activity in the olfactory bulb granule cell network
- PMID: 17093092
- PMCID: PMC6674772
- DOI: 10.1523/JNEUROSCI.3371-06.2006
Glomerulus-specific, long-latency activity in the olfactory bulb granule cell network
Abstract
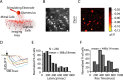
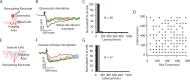
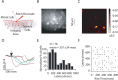
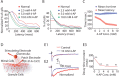
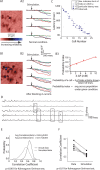
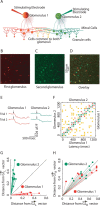
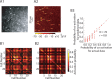
Reliable, stimulus-specific temporal patterns of action potentials have been proposed to encode information in many brain areas, perhaps most notably in the olfactory system. Analysis of such temporal coding has focused almost exclusively on excitatory neurons. Thus, the role of networks of inhibitory interneurons in establishing and maintaining this reliability is unclear. Here we use imaging of population activity in vitro to investigate the mechanisms of temporal pattern generation in mouse olfactory bulb inhibitory interneurons. We show that activity of these interneurons evolves slowly in time but that individual neurons fire at reliable times, with a timescale similar to the slow changes in the patterns of odor-evoked activity and to odor discrimination. Most strikingly, the latency of a single granule cell is highly reliable from trial to trial during repeated stimulation of the same glomerulus, whereas this same cell will have a markedly different latency when a different glomerulus is activated. These data suggest that the timing of granule cell-mediated inhibition in the olfactory bulb is tightly regulated by the source of input and that inhibition may contribute to the generation of reliable temporal patterns of mitral cell activity.
Figures

References
-
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. - PubMed
-
- Balu R, Larimer P, Strowbridge BW. Phasic stimuli evoke precisely timed spikes in intermittently discharging mitral cells. J Neurophysiol. 2004;92:743–753. - PubMed
-
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
