Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task
- PMID: 17093094
- PMCID: PMC6674769
- DOI: 10.1523/JNEUROSCI.3420-06.2006
Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task
Abstract
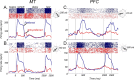
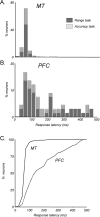
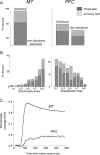
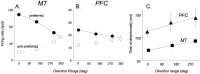
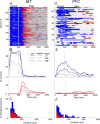
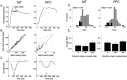
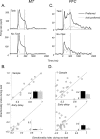
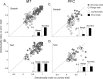
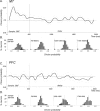
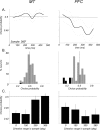
Neurons in the middle temporal visual area (MT) have been implicated in the perception of visual motion, whereas prefrontal cortex (PFC) neurons have been linked to temporary storage of sensory signals, attentional and executive control of behavior. Using a task that placed demands on both sets of neurons, we investigated their contribution to working memory for visual motion. Monkeys compared the direction of two moving random-dot stimuli, sample and test, separated by a brief memory delay. Neurons in both areas showed robust direction-selective activity during all phases of the task. During the sample, approximately 60% of task-related PFC neurons were direction selective, and this selectivity emerged 40 ms later than in MT. Unlike MT, the PFC responses to sample did not correlate with behavioral choices, but their selectivity was modulated by task demands and diminished on error trials. Reliable directional signals were found in both areas during the memory delay, but these signals were transient rather than sustained by neurons of either area. Responses to the test in both areas were modulated by the remembered sample direction, decreasing when the test direction matched the sample. This decrease arose in the PFC 100 ms later than in MT and was predictive of the forthcoming decision. Our data suggest that neurons in the two regions are functionally connected and make unique contributions to different task components. PFC neurons reflect task-related information about visual motion and represent decisions that may be based, in part, on the comparison in MT between the remembered sample and test.
Figures

References
-
- Amemori K-i, Sawaguchi T. Rule-dependent shifting of sensorimotor representation in the primate prefrontal cortex. Eur J Neurosci. 2006;23:1895–1909. - PubMed
-
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol. 2000;84:451–459. - PubMed
-
- Baddeley A. Oxford: Clarendon/Oxford UP; 1986. Working memory.
-
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. - PubMed
-
- Baker JF, Petersen SE, Newsome WT, Allman JM. Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus): a quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas. J Neurophysiol. 1981;45:397–416. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
