Dynamic spatial processing originates in early visual pathways
- PMID: 17093097
- PMCID: PMC6674796
- DOI: 10.1523/JNEUROSCI.3297-06.2006
Dynamic spatial processing originates in early visual pathways
Abstract
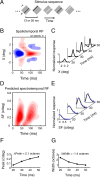
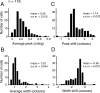
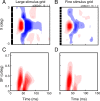
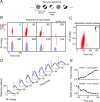
A variety of studies in the visual system demonstrate that coarse spatial features are processed before those of fine detail. This aspect of visual processing is assumed to originate in striate cortex, where single cells exhibit a refinement of spatial frequency tuning over the duration of their response. However, in early visual pathways, well known temporal differences are present between center and surround components of receptive fields. Specifically, response latency of the receptive field center is relatively shorter than that of the surround. This spatiotemporal inseparability could provide the basis of coarse-to-fine dynamics in early and subsequent visual areas. We have investigated this possibility with three separate approaches. First, we predict spatial-frequency tuning dynamics from the spatiotemporal receptive fields of 118 cells in the lateral geniculate nucleus (LGN). Second, we compare these linear predictions to measurements of tuning dynamics obtained with a subspace reverse correlation technique. We find that tuning evolves dramatically in thalamic cells, and that tuning changes are generally consistent with the temporal differences between spatiotemporal receptive field components. Third, we use a model to examine how different sources of dynamic input from early visual pathways can affect tuning in cortical cells. We identify two mechanisms capable of producing substantial dynamics at the cortical level: (1) the center-surround delay in individual LGN neurons, and (2) convergent input from multiple cells with different receptive field sizes and response latencies. Overall, our simulations suggest that coarse-to-fine tuning in the visual cortex can be generated completely by a feedforward process.
Figures



Similar articles
-
Functional consequences of neuronal divergence within the retinogeniculate pathway.J Neurophysiol. 2009 Apr;101(4):2166-85. doi: 10.1152/jn.91088.2008. Epub 2009 Jan 28. J Neurophysiol. 2009. PMID: 19176606 Free PMC article.
-
Orientation tuning of surround suppression in lateral geniculate nucleus and primary visual cortex of cat.Neuroscience. 2007 Nov 23;149(4):962-75. doi: 10.1016/j.neuroscience.2007.08.001. Epub 2007 Aug 9. Neuroscience. 2007. PMID: 17945429
-
Receptive-field maps of correlated discharge between pairs of neurons in the cat's visual cortex.J Neurophysiol. 1994 Jan;71(1):330-46. doi: 10.1152/jn.1994.71.1.330. J Neurophysiol. 1994. PMID: 8158235
-
Mapping the primate lateral geniculate nucleus: a review of experiments and methods.J Physiol Paris. 2014 Feb;108(1):3-10. doi: 10.1016/j.jphysparis.2013.10.001. Epub 2013 Nov 21. J Physiol Paris. 2014. PMID: 24270042 Free PMC article. Review.
-
Dynamic properties of thalamic neurons for vision.Prog Brain Res. 2005;149:83-90. doi: 10.1016/S0079-6123(05)49007-X. Prog Brain Res. 2005. PMID: 16226578 Review.
Cited by
-
Transcranial Magnetic Stimulation Changes Response Selectivity of Neurons in the Visual Cortex.Brain Stimul. 2015 May-Jun;8(3):613-23. doi: 10.1016/j.brs.2015.01.407. Epub 2015 Jan 24. Brain Stimul. 2015. PMID: 25862599 Free PMC article.
-
From coarse to fine? Spatial and temporal dynamics of cortical face processing.Cereb Cortex. 2011 Feb;21(2):467-76. doi: 10.1093/cercor/bhq112. Epub 2010 Jun 24. Cereb Cortex. 2011. PMID: 20576927 Free PMC article.
-
Coarse-to-fine interaction on perceived depth in compound grating.J Vis. 2023 Oct 4;23(12):5. doi: 10.1167/jov.23.12.5. J Vis. 2023. PMID: 37856108 Free PMC article.
-
Effects of cortical feedback on the spatial properties of relay cells in the lateral geniculate nucleus.J Neurophysiol. 2013 Feb;109(3):889-99. doi: 10.1152/jn.00194.2012. Epub 2012 Oct 24. J Neurophysiol. 2013. PMID: 23100142 Free PMC article.
-
Push-Pull Receptive Field Organization and Synaptic Depression: Mechanisms for Reliably Encoding Naturalistic Stimuli in V1.Front Neural Circuits. 2016 May 11;10:37. doi: 10.3389/fncir.2016.00037. eCollection 2016. Front Neural Circuits. 2016. PMID: 27242445 Free PMC article.
References
-
- Albrecht DG. Visual cortex neurons in monkey and cat: effect of contrast on the spatial and temporal phase transfer functions. Vis Neurosci. 1995;12:1191–1210. - PubMed
-
- Anzai A, Ohzawa I, Freeman RD. Neural mechanisms for encoding binocular disparity: receptive field position versus phase. J Neurophysiol. 1999;82:874–890. - PubMed
-
- Bauman LA, Bonds AB. Inhibitory refinement of spatial frequency selectivity in single cells of the cat striate cortex. Vision Res. 1991;31:933–944. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
