Properties of somatosensory synaptic integration in cerebellar granule cells in vivo
- PMID: 17093099
- PMCID: PMC6674774
- DOI: 10.1523/JNEUROSCI.2939-06.2006
Properties of somatosensory synaptic integration in cerebellar granule cells in vivo
Abstract
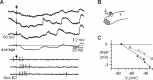
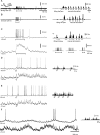
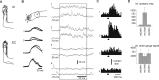
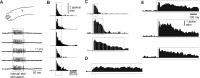
In decerebrated, nonanesthetized cats, we made intracellular whole-cell recordings and extracellular cell-attached recordings from granule cells in the cerebellar C3 zone. Spontaneous EPSPs had large, relatively constant peak amplitudes, whereas IPSPs were small and did not appear to contribute substantially to synaptic integration at a short time scale. In many cases, the EPSPs of individual mossy fiber synapses appeared to be separable by their peak amplitudes. A substantial proportion of our granule cells had small receptive fields on the forelimb skin. Skin stimulation evoked explosive responses in which the constituent EPSPs were analyzed. In the rising phase of the response, our analyses indicated a participation of three to four different mossy fiber synapses, corresponding to the total number of mossy fiber afferents. The cutaneous receptive fields of the driven EPSPs overlapped, indicating an absence of convergence of mossy fibers activated from different receptive fields. Also in granule cells activated by joint movements did we find indications that different afferents were driven by the same type of input. Regardless of input type, the temporal patterns of granule cell spike activity, both spontaneous and evoked, appeared to primarily follow the activity in the presynaptic mossy fibers, although much of the nonsynchronized mossy fiber input was filtered out. In contrast to the prevailing theories of granule cell function, our results suggest a function of granule cells as signal-to-noise enhancing threshold elements, rather than as sparse coding pattern discriminators or temporal pattern generators.
Figures

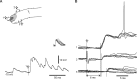



References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage- independent potassium conductance. Nature. 2001;409:88–92. - PubMed
-
- Brodal P, Bjaalie JG. Organization of the pontine nuclei. Neurosci Res. 1992;13:83–118. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
