Remapping in human visual cortex
- PMID: 17093130
- PMCID: PMC2292409
- DOI: 10.1152/jn.00189.2006
Remapping in human visual cortex
Abstract
With each eye movement, stationary objects in the world change position on the retina, yet we perceive the world as stable. Spatial updating, or remapping, is one neural mechanism by which the brain compensates for shifts in the retinal image caused by voluntary eye movements. Remapping of a visual representation is believed to arise from a widespread neural circuit including parietal and frontal cortex. The current experiment tests the hypothesis that extrastriate visual areas in human cortex have access to remapped spatial information. We tested this hypothesis using functional magnetic resonance imaging (fMRI). We first identified the borders of several occipital lobe visual areas using standard retinotopic techniques. We then tested subjects while they performed a single-step saccade task analogous to the task used in neurophysiological studies in monkeys, and two conditions that control for visual and motor effects. We analyzed the fMRI time series data with a nonlinear, fully Bayesian hierarchical statistical model. We identified remapping as activity in the single-step task that could not be attributed to purely visual or oculomotor effects. The strength of remapping was roughly monotonic with position in the visual hierarchy: remapped responses were largest in areas V3A and hV4 and smallest in V1 and V2. These results demonstrate that updated visual representations are present in cortical areas that are directly linked to visual perception.
Figures

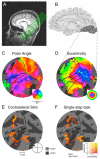
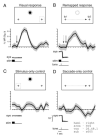



Comment in
-
From a different point of view: extrastriate cortex integrates information across saccades. Focus on "Remapping in human visual cortex".J Neurophysiol. 2007 Feb;97(2):961-2. doi: 10.1152/jn.01225.2006. Epub 2006 Dec 6. J Neurophysiol. 2007. PMID: 17151216 No abstract available.
References
-
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. - PubMed
-
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. - PubMed
-
- Birn RM, Bandettini PA. The effect of stimulus duty cycle and “off” duration on BOLD response linearity. Neuroimage. 2005;27:70–82. - PubMed
-
- Birn RM, Saad ZS, Bandettini PA. Spatial heterogeneity of the nonlinear dynamics in the fMRI BOLD response. Neuroimage. 2001;14:817–826. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

