The adenovirus L4 33-kilodalton protein binds to intragenic sequences of the major late promoter required for late phase-specific stimulation of transcription
- PMID: 17093188
- PMCID: PMC1797539
- DOI: 10.1128/JVI.01584-06
The adenovirus L4 33-kilodalton protein binds to intragenic sequences of the major late promoter required for late phase-specific stimulation of transcription
Abstract
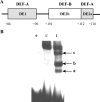
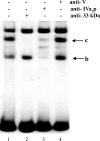
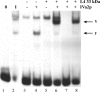
The adenovirus late IVa2 protein is required for maximally efficient transcription from the viral major late (ML) promoter, and hence, the synthesis of the majority of viral late proteins. This protein is a sequence-specific DNA-binding protein that also promotes the assembly of progeny virus particles. Previous studies have established that a IVa2 protein dimer (DEF-B) binds specifically to an intragenic ML promoter sequence necessary for late phase-specific stimulation of ML transcription. However, activation of transcription from the ML promoter correlates with binding of at least one additional infected-cell-specific protein, termed DEF-A, to the promoter. Using an assay for the DNA-binding activity of DEF-A, we identified the unknown protein by using conventional purification methods, purification of FLAG-tagged IVa2-protein-containing complexes, and transient synthesis of viral late proteins. The results of these experiments established that the viral L4 33-kDa protein is the only component of DEF-A: the IVa2 and L4 33-kDa proteins are necessary and sufficient for formation of all previously described complexes in the intragenic control region of the ML promoter. Furthermore, the L4 33-kDa protein binds to the promoter with the specificity characteristic of DEF-A and stimulates transcription from the ML promoter in transient-expression assays.
Figures





References
-
- Akusjärvi, G., P. Aleström, M. Pettersson, M. Lager, H. Jornvall, and U. Pettersson. 1984. The gene for the adenovirus 2 hexon polypeptide. J. Biol. Chem. 259:13976-13979. - PubMed
-
- Akusjärvi, G., and H. Persson. 1981. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature 292:420-426. - PubMed
-
- Axelrod, N. 1978. Phosphoproteins of adenovirus 2. Virology 87:366-383. - PubMed
-
- Beltz, G. A., and S. J. Flint. 1979. Inhibition of HeLa cell protein synthesis during adenovirus infection: restriction of cellular messenger RNA sequences to the nucleus. J. Mol. Biol. 131:353-373. - PubMed
-
- Bentley, D. 1999. Coupling RNA polymerase II transcription with pre-mRNA processing. Curr. Opin. Cell Biol. 11:347-351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

